Virtual Library
Start Your Search
Pierre P Massion
Author of
-
+
MA23 - Early Stage Lung Cancer: Present and Future (ID 926)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment of Early Stage/Localized Disease
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 105
-
+
MA23.03 - Risk Assessment for Indeterminate Pulmonary Nodules Using a Novel, Plasma-Protein Based Biomarker Assay (ID 13873)
10:40 - 10:45 | Author(s): Pierre P Massion
- Abstract
- Presentation
Background
To reduce overdiagnosis and overtreatment of non-cancerous pulmonary nodules found on CT scans, a noninvasive and easily administered test is needed to assess clinically significant disease risk. Such an assay should also accurately inform whether additional aggressive evaluation, including lung biopsy or thoracic surgery, is warranted.
Objective: To determine the performance of a novel, plasma-based multiplexed protein test model when compared to the Veterans Affairs Clinical Factors Model (VA model) for discriminating between a lung cancer diagnosis established pathologically and an Indeterminate Pulmonary Nodule (IPN) found to be clinically and radiographically stable for at least one year.
a9ded1e5ce5d75814730bb4caaf49419 Method
The protein biomarker-based risk model had been trained and tested with a cohort of 277 subjects at high risk of lung cancer, aged 25-85, who were current smokers with an indeterminate lung nodule 4-30mm in diameter (121 subject training set; 59 subject test set) from eight medical centers across the US. Using retrospective plasma samples, we compared the protein biomarker model results with the malignant or benign outcomes in an independent validation cohort comprised of 97 subjects from the Vanderbilt University medical center.
4c3880bb027f159e801041b1021e88e8 Result
Among the 97 validation study subjects (average age 60.1 years, range 42-83; average nodule size 16.1mm), the protein biomarker model correctly identified as benign or malignant an additional 44 of the 68 (65%) indeterminate pulmonary nodules classified as having intermediate risk by the VA model. Negative predictive value was 0.94. Only three patients with malignant disease were missed (94% sensitivity) while an additional 28 intermediate risk samples (41%) were properly classified as true positive, thus potentially avoiding aggressive interventions in those subjects with benign disease.
8eea62084ca7e541d918e823422bd82e Conclusion
This study evaluated a novel plasma protein biomarker assay model as a noninvasive risk assessment aid for characterizing indeterminate pulmonary nodules. When the model results are combined with the VA model, risk stratification for benign nodules is improved compared to current methods in clinical practice. We hypothesize patients with benign disease may benefit the most from this assay by avoiding unnecessary lung biopsy and subsequent overtreatment, while improving patient quality of care and reducing risks from these procedures. Providers and their patients in whom they suspect lung cancer may consider using this novel assay prior to proceeding with more aggressive interventions.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P2.10 - Prevention and Tobacco Control (Not CME Accredited Session) (ID 959)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.10-05 - Provider Medical Specialty Influences Smoking Cessation Counseling at an Academic Medical Center and Veterans Affairs in U.S. Mid-South (ID 12820)
16:45 - 18:00 | Author(s): Pierre P Massion
- Abstract
Background
Tobacco accounts for 11.5% of deaths worldwide and approximately 30% of U.S. cancer deaths. Smoking cessation counseling using the 5 A’s (Ask, Advise, Assess, Assist, Arrange) is standard-of-care for all smokers. We tested the hypothesis that certain provider characteristics (general internal medicine [GIM], those with lung cancer screening [LCS] knowledge, and those who perceive smoking cessation as very effective) would be associated with smoking cessation counseling.
a9ded1e5ce5d75814730bb4caaf49419 Method
We surveyed all GIM, pulmonology, hematology/oncology, and gynecology providers (physicians/advanced practice providers) at a large academic institution in the Mid-South and affiliated VA from February to May 2017. The primary predictor variables were provider characteristics including: specialty, LCS guideline knowledge (high knowledge=identified start age 55, 30 pack-years, current & former smokers), and perceived effectiveness of smoking cessation, colonoscopy, and pap smear at reducing cancer mortality (very/moderately/minimally/not effective). The primary outcome was self-reported smoking cessation counseling using the 5 A’s within the past year. Data were summarized using counts, proportions, and medians. We used multivariable logistic regression adjusted for years in practice and healthcare system to evaluate the association of medical specialty with counseling. Logistic regressions of perceived effectiveness and LCS guideline knowledge as predictors of counseling were further adjusted for specialty.
4c3880bb027f159e801041b1021e88e8 Result
Of 625 providers invited, 407 (65%) responded, 378 (60.5%) questionnaires were analyzed, of which 58% were GIM, 19% hematology/oncology, 13% gynecology, 10% pulmonology. There were 255 of 350 providers (73%) who reported performing smoking cessation counseling within the past year (77% GIM vs 77% pulmonology vs 57% hematology/oncology vs 72% gynecology, χ2 p=0.013). In adjusted multivariable logistic regression, GIM (aOR 2.52 95% CI 1.40,4.54;p=0.002) and pulmonology (aOR 2.52 95% CI 1.00,6.36;p=0.05) providers were more likely to perform smoking cessation counseling than hematology/oncology providers.
41% vs 59% of those who provided counseling had high LCS knowledge vs low LCS knowledge. Fewer providers (71%) reported smoking cessation as very effective at reducing cancer-specific mortality compared to colonoscopy (77%) and pap-smear (74%). Perceived effectiveness and high LCS guideline knowledge did not predict smoking cessation counseling (aOR 1.1 95% CI 0.64,1.8;p=0.78;aOR 1.2 95% CI 0.72,2.0 p=0.47 respectively).
8eea62084ca7e541d918e823422bd82e Conclusion
Providers in general internal medicine and pulmonology were more likely than those in hematology/oncology to report performing smoking cessation counseling. Perceived effectiveness and LCS guideline knowledge did not predict smoking cessation counseling. Targeted interventions, especially in hematology/oncology, are needed to increase smoking cessation counseling using the 5 A’s. Future education should also address knowledge gaps in smoking cessation effectiveness and LCS guidelines.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.11 - Screening and Early Detection (Not CME Accredited Session) (ID 960)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.11-06 - A New Histoplasmosis Antibody Enzyme Immunoassay for the Diagnosis of Lung Benign Nodules (ID 13881)
16:45 - 18:00 | Author(s): Pierre P Massion
- Abstract
Background
Granulomas caused by infectious lung diseases often present as indeterminate pulmonary nodules on radiography. Newly available antibody enzyme immunoassay (EIA) test for histoplasmosis has not been studied for the evaluation of lung nodules suspicious for cancer. This study investigates serum biomarkers for histoplasmosis measured by a new CLIA-certified test as a response to exposure when positive and as a predictor of benign disease in indeterminate pulmonary lung nodules from a highly endemic region.
a9ded1e5ce5d75814730bb4caaf49419 Method
376 serum samples from our biorepository of patients presenting with pulmonary nodules ≤30mm in maximum diameter were analyzed for histoplasmosis by the IgG and IgM EIA. Manufacturer-suggested levels for histoplasmosis diagnosis was used to indicate a positive test for antibodies. Final diagnosis was determined pathologically or by radiographic follow-up. Diagnostic test characteristics with 95% confidence intervals (CI) for benign lung disease were estimated.
4c3880bb027f159e801041b1021e88e8 Result
Cancer prevalence was 59% (n=223). Seventy-seven (20%) samples were positive for IgG anti-Histoplasma antibodies (Table 1). Twenty-one (5.6%) were IgM antibody positive and 84 (22%) were positive for either antibody. Positive diagnostic likelihood ratios (DLR) for benign disease were 3.22 (95%CI: 2.08,4.98) for IgG antibodies, 6.19 (95%CI: 2.13,18.1) for IgM antibodies each separately, and 2.92 (95%CI: 1.95,4.37) if either IgG or IgM were positive. When both IgG and IgM antibodies were positive then DLR was 42.2 (95%CI: 2.54,702). When both IgG and IgM were found (n=14), no nodules were cancerous. Only a positive EIA test offered clinically informative results.
8eea62084ca7e541d918e823422bd82e Conclusion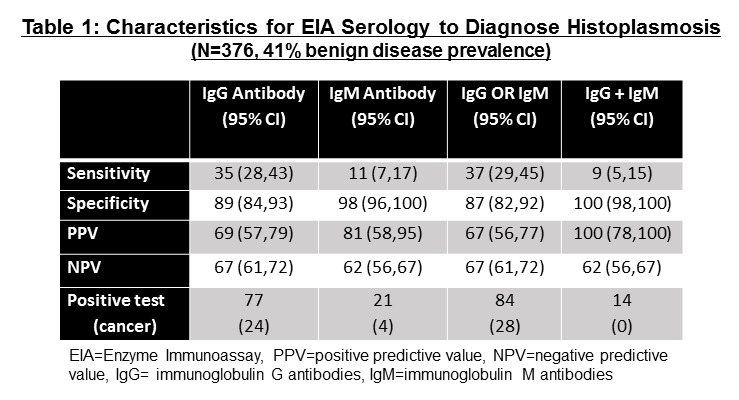
A positive EIA test for both IgM and IgG strongly suggests benign granuloma and ruled out lung cancer for 9% of benign nodules arising from a highly endemic area of the country. The new EIA test may improve the diagnostic evaluation of indeterminate pulmonary nodules and avoid harmful invasive biopsies. Additional investigation in conjunction with other non-invasive cancer biomarker tests is warranted.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.11-10 - Potential Utility of a Positive EarlyCDT®-Lung Blood Biomarker Test in Indeterminate Pulmonary Nodules (ID 14028)
16:45 - 18:00 | Author(s): Pierre P Massion
- Abstract
Background
EarlyCDT®-Lung is a blood-based biomarker of risk for lung cancer and is able to detect early stage disease. Indeterminate pulmonary nodules (IPN) of 8-20 mm are a common clinical problem. Approximately 20% of IPNs are malignant in many series, but determining which ones are malignant versus benign is a dilemma. The ACCP guidelines recommend surgical resection for IPN of high risk for malignancy (>65%). A moderate positive EarlyCDT-Lung (sensitivity 40%; specificity 93%) has been shown to increase the risk of malignancy in IPNs of intermediate risk (10-65%) (Massion et al J Thorac Oncol 2017).
a9ded1e5ce5d75814730bb4caaf49419 Method
We evaluated 48 patients from a clinical audit with an IPN and a calculated risk of malignancy of >30% based on the Mayo/Swensen calculator.
4c3880bb027f159e801041b1021e88e8 Result
Twenty-five patients had benign nodules and 23 were proven to be lung cancer. The positive predictive value (PPV) of the calculator alone was 48%. The biomarker test was positive in 23% (11 of 48) of the population (>30% risk). Ten of these 11 were proven to be lung cancer and one was benign; PPV of 91%. This 9% rate for benign disease is a marked improvement from the average resection rate of 20-25% for benign disease that has been reported in multiple lung cancer screening trials (24% in NLST).
8eea62084ca7e541d918e823422bd82e Conclusion
Combining the EarlyCDT-Lung blood test with calculated risk of IPNs of >30% resulted in a high PPV and would result in a resection rate of IPNs for benign disease of <10%. A prospective clinical utility study with EarlyCDT-Lung and IPNs is planned.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.11 - Screening and Early Detection (Not CME Accredited Session) (ID 977)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.11-23 - Adherence to Annual Low-Dose CT Lung Cancer Screening at a Large Academic Institution (ID 12997)
12:00 - 13:30 | Author(s): Pierre P Massion
- Abstract
Background
Annual low-dose computed tomography (LDCT) is standard-of-care in the high-risk population for lung cancer screening. We assessed adherence to annual LDCT screening in a large academic institution.
a9ded1e5ce5d75814730bb4caaf49419 Method
We assembled a retrospective cohort of patients who underwent LDCT between January 1, 2014 and September 30, 2016.
We included patients with baseline LungRads 1 or 2 with 12-month follow-up recommendation. We excluded those who died before the time of recommended follow-up. The time interval between the recommended follow-up study date and the actual follow-up study was calculated. Patient adherence at time of due follow-up was defined by this time interval: <=90 days (adherent) and >90 days (non-adherent).
Primary analysis was change in adherence over time. Secondary analysis included descriptive statistics of demographics.
4c3880bb027f159e801041b1021e88e8 Result

395 patients had baseline LDCT screening. We found a persistent, significant increase in adherence: 11% (2/18) in the 1st quarter 2015 and 70% (46/66) in the 3rd quarter 2017 (Pearson’s chi-squared test p=.008). We also identified racial disparity in patients enrolling in our Lung Screening Program (93% white).
8eea62084ca7e541d918e823422bd82e Conclusion
Adherence to annual LDCT screening significantly increased from 2015 to 2017. Improvement may be due to changes in national policy and/or implementation of a dedicated program physician director and program coordinator. Future work should address racial disparities and barriers to and facilitators of annual LDCT screening.
6f8b794f3246b0c1e1780bb4d4d5dc53


