Virtual Library
Start Your Search
Yan Wang
Author of
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-101 - Survival Benefit of Sequential Therapy in ALK Positive Olioprogressive NSCLC Patients After Crizotinib Resistance (ID 13110)
16:45 - 18:00 | Author(s): Yan Wang
- Abstract
Background
Crizotinib is recommended as first-line standard therapy for advanced anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC).Despite initial efficacy of crizotinib, patients ultimately acquired resistance, which leading to tumor progression within one year of crizotinib initiation. However,optimal sequential therapy option among patients with olioprogressive disease remains unknown.Therefore, this study aimed to explore which therapy pattern was advantageous for those patients with extracranial and intracranial metastasis.
a9ded1e5ce5d75814730bb4caaf49419 Method
This retrospective single-center study enrolled 116 Chinese advanced ALK positive NSCLC patients resistant to prior crizotinib treatment,between Jan 2013 and Dec 2016.Based on the site of olioprogressive metastasis, patients were divided into two groups: extracranial (n=57,49.1%) and intracranial (n=59, 50.9%).Sequential therapies mainly included continuation of crizotinib with local therapeutics and next-generation ALK inhibitors. The primary end-point was median overall survival (OS) from the time of crizotinib resistance to death or last follow-up.
4c3880bb027f159e801041b1021e88e8 Result
Among 116 patients,45 patients (38.8%) had died. For extracranial group,26 patients continued crizotinib with local treatment (local radiotherapy or ablation therapy),31 received next-generation ALK inhibitor. Patients followed by next-generation ALK inhibitor beyond crizotinib resistance exhibited a longer OS of 27.6 months than crizotinib continuation with local therapy of 10.8months(p=0.009).Among intracranial group,36 patients continued crizotinib with local treatment including whole brain radiotherapy or stereotactic radiotherapy,23 were next-generation ALK inhibitors users. Continution of crizotinib with local therapy had a non-inferior OS than next-generation ALK inhibitors (28.9 month vs 32.8months, p=0.538) .
8eea62084ca7e541d918e823422bd82e Conclusion
Next-generation ALK inhibitors had more survival benefit than continuation of crizotinib with local therapy for extracranial olioprogressive patients.While crizotinib continuation with local therapy might be a feasible strategy among patients with intracranial olioprogressive metastasis after crizotinib resistance.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 944)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.12-03 - A Prospective Study of Apatinib in Advanced Small Cell Lung Cancer Patients Failed from Two or More Lines of Chemotherapy (ID 13140)
16:45 - 18:00 | Author(s): Yan Wang
- Abstract
Background
Small lung cancer (SCLC), a highly malignant neoplastic, chemoresponsive disease. For SCLC patients who with worsening status after second-line treatment, there is currently no affirmative and widely accepted chemotherapy regimen. Apatinib is a novel oral multi-target small-molecule TKI mainly targeting the intracellular ATP-binding domain of VEGFR-2, which has a significant effect of anti-angiogenesis to suppress the growth of tumors. This study evaluated the efficacy and safety of Apatinib in SCLC patients who failed from second- or further-line chemotherapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Data was collected from the files of patients treated with Apatinib 500mg qd who diagnosed with advanced SCLC and failed from second or more lines of chemotherapy. Efficacy assessed after one cycles (4 weeks), then every two cycles (8 weeks) once again. The primary endpoint was PFS and the tumor response was determined according to the RECIST 1.1. PFS were graphed by Kaplan-Meier curves of progression-free survival. AEs were also evaluated and toxicity grade was determined based on CTCAE 4.0.
4c3880bb027f159e801041b1021e88e8 Result
22 patients were enrolled from November 10, 2016 to April 18, 2018, the number of patients that can be evaluated is 19. One patients obtained partial response, and 15 obtained stable disease, representing a DCR of 84.11%. Median PFS was 140 days (95% confidence interval [CI] 94.84–185.16). Although only one patient showed PR, all the patients’ target lesions were reduced. A total of 46 AEs were reported during the trial, grade 3-4 AEs were hypertension (9.09%), leukopenia (4.55%) and proteinuria (4.55%) which most could be relieved by dose reduction.
8eea62084ca7e541d918e823422bd82e Conclusion
In conclusion, Apatinib has a certain therapeutic effect in patients with advanced SCLC (third- or further-line). To further investigate the role of Apatinib in advanced SCLC patients, large sample and additional clinical trials are needed.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-109 - Treatment Duration—A More Reasonable Definition to Evaluate the Efficacy of Crizotinib in ALK Positive Advanced NSCLC (ID 13216)
16:45 - 18:00 | Author(s): Yan Wang
- Abstract
Background
Crizotinib has demonstrated its superior efficacy in ALK positive NSCLC patients when used as first-line regimen, with a median overall survival (OS) of more than 4 years, whereas a median progression-free survival (PFS) of only 10.9 months. Patients who continued crizotinib beyond progressive disease (CBPD) could still obtain additional survival benefits of 6-8 months after disease progression. In terms of that, PFS, the frequently used primary endpoint in clinical trials, may not be able to provide accurate information on impact of this intervention in multiple lines therapy. Here we proposed “treatment duration” as an intermediate clinical endpoint between PFS and OS that further define efficacy of crizotinib in multiple lines of treatment and reported the exploratory data in a real-world cohort.
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively enrolled 150 ALK positive NSCLC patients who had acquired crizotinib resistance from Aug 2011 to May 2017. The median PFS of crizotinib and median OS (from crizotinib initiation) were analyzed. Treatment duration of crizotinib, the time from crizotinib initiation to discontinuation, was also calculated.
4c3880bb027f159e801041b1021e88e8 Result
The median PFS of crizotinib in the 150 ALK positive advanced NSCLC patients was 14.4 months (95% CI: 11.3-17.4). Overall, 58 patients (38%) continued CBPD and the median post-progression PFS was 10.4 months, resulting in a median treatment duration of crizotinib in the total cohort of 20.2 months (95% CI: 14.3-26.0). And median OS was 30.1months (95% CI: 21.3-38.8). 77 (51%) patients who received crizotinib as first-line therapy exhibited both longer median PFS (17.7 months, 95% CI: 12.5-22.9; vs. 12.2months, 95% CI: 7.6-16.8) and median OS (35.2 months, 95% CI: 23.2-47.1; vs. 25.4 months, 95% CI: 14.4-36.3) compared to those selected crizotinib as second- line and above therapy. The treatment duration among them were 23.1 months (95% CI: 14.7-31.4) and 18.9 months (95% CI: 11.4-26.3), respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
Crizotinib showed superior efficacy in ALK positive NSCLC patients. Treatment duration may be more reasonable to define the efficacy of crizotinib in multiple lines therapy of ALK positive NSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.13 - Targeted Therapy (Not CME Accredited Session) (ID 962)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.13-04 - Outcomes of ALK-Positive Non-Small-Cell Lung Cancer (NSCLC) Patients Treated with Crizotinib: A Multicenter Cohort Retrospective Study. (ID 13304)
16:45 - 18:00 | Author(s): Yan Wang
- Abstract
Background
Crizotinib has demonstrated promising efficacy in patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) in clinical trials. However, there is lack of multicenter real-world data summary with large cohort of patients, especially in China. We conducted this multicenter and retrospective study to assess the outcomes of crizotinib in, to our knowledge, the largest cohort of patients with ALK-positive advanced NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
We reviewed medical records of 484 unselected ALK-positive NSCLC patients treated with crizotinib at five cancer centers in China from January 2013 to November 2017. Clinical data were collected from crizotinib initiation to RECIST-defined progressive disease (PD), and post-PD systemic treatment outcomes were also analyzed.
4c3880bb027f159e801041b1021e88e8 Result
A total of 428 eligible ALK-positive NSCLC patients were enrolled. Among them, 273 (63.8%) patients received crizotinib as first-line treatment. The median progression-free survival (PFS) and overall survival (OS) from crizotinib initiation were 14.4 months (95%CI: 12.4-16.4) and 53.4 months (95% CI: 33.7-73.1) respectively. In the subgroup analysis, patients received crizotinib as first-line treatment showed a higher disease control rate (DCR) and longer median OS with statistical significance compared with second-/ further line crizotinib treatment (94.8%, not estimated vs. 89.0%, 40.5 months, respectively). For 261 patients with RECIST-defined PD, multivariate COX analysis revealed that patients who received first-line crizotinib (P=0.013), continued crizotinib beyond progressive disease (CBPD) (P=0.011) and received next-generation ALKis after crizotinib failure (P<0.001) were associated with improved survival both from crizotinib progression and from the first crizotinib dose.
8eea62084ca7e541d918e823422bd82e Conclusion
This study demonstrated the clinically meaningful benefit of crizotinib treatment in the largest cohort of Chinese ALK positive NSCLC patients. CBPD and next-generation ALK TKI treatment may provide survival improvement after RECIST-defined progression on crizotinib.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.13-05 - Real-World Clinical Benefit of Continuing Crizotinib Beyond Progression Disease (CBPD) in Patients with Advanced ALK-Positive NSCLC. (ID 13353)
16:45 - 18:00 | Author(s): Yan Wang
- Abstract
Background
Most ALK-positive NSCLC patients treated with crizotinib would ultimately develop progressive disease (PD), and continuing crizotinib beyond initial PD (CBPD) may be potentially beneficial. We aim to evaluate the survival outcomes of patients with crizotinib resistance in real-world setting and to explore the clinical efficacy of continuing CBPD treatment.
a9ded1e5ce5d75814730bb4caaf49419 Method
A total of 261 ALK-positive NSCLC patients treated with crizotinib experienced RECIST-defined PD and were included in this multi-center retrospective analysis. Clinicopathologic characteristics, progressive pattern, post-PD treatment and overall survival (OS) were compared between patients continuing CBPD and those not.
4c3880bb027f159e801041b1021e88e8 Result
140 patients who continued crizotinib after disease progression were allocated to CBPD group and others were non-CBPD group. Two-sided Chi-square test showed that patients who never smoked (P=0.047), with ECOG 0-1(P=0.001), isolated intracranial progression (P<0.001) and <median PFS of initial crizotinib (P=0.002) were more likely in the CBPD group. At the analysis, 84 patients had re-PD and the median duration of crizotinib treatment post-PD was 6.8 months (95%CI: 3.639-9.869). The median OS for the overall population from the time of PD (post-PD OS) was 15.3 months (95%CI: 11.376-19.181), and was significantly longer in CBPD patients than non-CBPDs (24.1 months vs. 8.5 months, 95% CI: 0.326-0.669, HR 0.467, P<0.001). Furthermore, next-generation ALK inhibitors (ALKis) following crizotinib failure was associated with improved post-PD OS (24.9 months vs. 10.7 months, 95% CI: 0.307-0.686, HR 0.459, P<0.001).
8eea62084ca7e541d918e823422bd82e Conclusion
Continuing CBPD treatment after crizotinib resistance favorably impact survival outcomes of advanced ALK-positive NSCLC patients in the real-world. Next-generation ALKis may provide survival improvement, but comparative studies between different subsequent treatment options after PD on crizotinib are still needed.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-65 - First-Line Radical Local Therapy May Provide Additional Survival Gain for Patients with EGFR-Mutant Metastatic NSCLC Receiving TKIs (ID 12140)
12:00 - 13:30 | Author(s): Yan Wang
- Abstract
Background
Tyrosine kinase inhibitors (TKIs) have been widely accepted as first-line therapy for patients with EGFR-mutant metastatic non-small cell lung cancer (NSCLC), achieving a median progression free survival (PFS) and overall survival (OS) of 8.0 to 13.1 months and 19.3 to 30.9 months respectively. Patterns-of-failure studies suggest that the first progression after first-line TKIs occurs most often at sites of disease known to exist at the baseline. In this retrospective study, we aimed to investigate whether the radical local therapy could offer survival benefit in addition to st
a9ded1e5ce5d75814730bb4caaf49419 Method
NSCLC patients with activating EGFR mutations and treated with TKIs as first-line management after the diagnosis of stage IV disease (either synchronous or metachronous) between 2010 and 2017 at our institution were reviewed. All enrolled patients should receive radical local therapy (either surgery or radiotherapy with curative intent) at least to the main site of disease. We defined the main site as the primary site for synchronous IV-stage patients and at least 1 progression site for metachronous IV-stage patients. OS and PFS were calculated from the first day of IV-stage treatment. Kaplan-Meier method was used for survival estimation and Log-rank test was rendered for survival comparison between groups.
4c3880bb027f159e801041b1021e88e8 Result
A total of 45 patients entered into the final analysis, including 18with synchronous stage IV diseases and 27patients with metachronous diseases. A total of 208 gross tumor sites were identified and 130 of them received local treatment, including 90 sites treated with radical approaches and another 30 sites with palliative therapy. At a median follow-up period of 37.8months, the median OS was 51.4 months, with 1-, 3- and 5-year rate of 97.7%, 58.7% and 25.2%, respectively. The median PFS was 16.4months, with 1-, 2- and 3-year rate of 64.7%, 29.1% and 6.8%, respectively. There was no difference between synchronous and metachronous groups. In 38patients who progressed, 19 (42.2%) involved new metastatic sites only, 12(26.6%) involved initial sites only, and 3 (6.7%) involved both.
8eea62084ca7e541d918e823422bd82e Conclusion
Metastatic EGFR-mutant NSCLC patients who received TKIs and radical local therapy in our study obviously provided longer OS and PFS compared with historical results using TKIs alone. Prospective randomized evidences are warranted to clarify the clinical efficacy of additional local therapy to first-line TKIs for this highly selective subgroup of patients.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.13 - Targeted Therapy (Not CME Accredited Session) (ID 979)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.13-17 - A Retrospective Study: Central Nervous System Response to Osimertinib in Patients with Advanced NSCLC (ID 11937)
12:00 - 13:30 | Author(s): Yan Wang
- Abstract
Background
Central nervous system (CNS) metastases are common in patients with non-small-cell lung cancer (NSCLC). More than 30% of patients who progress during or after treatment with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have CNS metastases. Osimertinib, a third-generation EGFR-TKI, has been demonstrated promising intracranial efficacy in patients with advanced NSCLC from several large scale randomized control trials. We aimed to explore clinical impact of osimertinib for patients with CNS metastases, advanced NSCLC in real world setting.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with advanced NSCLC who received osimertinib after progression of prior EGFR-TKIs and CNS metastases on baseline brain scan were retrospectively collected from Cancer Hospital Chinese Academy of Medical Sciences. Primary outcome was objective response rate (ORR) and secondary objectives were disease control rate (DCR), progression-free survival (PFS), time to tumor response, median best percentage change from baseline in CNS target lesion (TL) size and safety.
4c3880bb027f159e801041b1021e88e8 Result
Between Apr 1, 2017, and Dec 30, 2017, 22 patients met selection criteria, 15 with ≥1 measurable CNS lesion (RECIST 1.1) were included in CNS evaluable for response (cEFR) set. The median duration of follow-up was 6.5 months. For overall 22 patients, ORR and DCR were 40.9% and 86.4%, respectively, with median PFS of 8.5 months (95% CI 4.1, 13.0). Of 15 patients in cEFR set, CNS ORR was 53.3% with complete responses reported in 3 patients (20.0%). Median best percentage change from baseline in CNS TL size was -40% (range: -100% ~ +60%) and median time to CNS tumor response was 1.3 months. CNS DCR was 80.0%. Median CNS PFS was not reached. Safety profile was acceptable and no new unexpected findings were found.
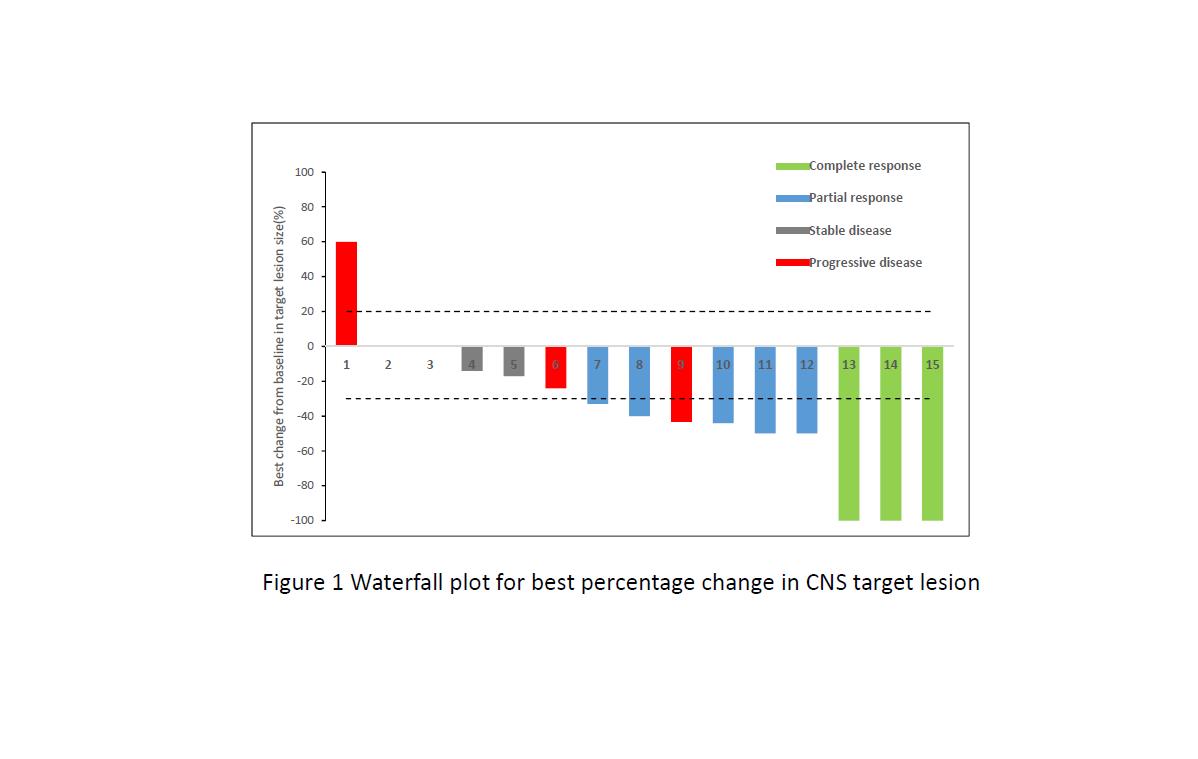
8eea62084ca7e541d918e823422bd82e Conclusion
This real world analysis further confirmed that osimertinib was indeed demonstrated clinically meaningful efficacy against CNS metastases in Chinese patients with advanced NSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53


