Virtual Library
Start Your Search
Tetsuya Mitsudomi
Author of
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-34 - Docetaxel Plus Ramucirumab with Prophylactic PEG-G-CSF Support for Chemo-NaïVe Elderly NSCLC Patients: A Phase II Study (WJOG9416L) (ID 12400)
16:45 - 18:00 | Author(s): Tetsuya Mitsudomi
- Abstract
Background
Docetaxel monotherapy is the standard of care for chemo-naïve Japanese elderly patients with advanced non-small cell lung cancer (NSCLC), according to our results of phase III trial comparing docetaxel and vinorelbine monotherapies (WJTOG9904). In a pivotal phase III study (REVEL), docetaxel plus ramucirumab demonstrated superior response rate (RR) and progression-free survival (PFS) over docetaxel monotherapy in second-line setting for advanced NSCLC. These differences in RR and PFS were translated into overall survival (OS) benefit. This evidence prompted us to investigate docetaxel plus ramucirumab for chemo-naïve elderly patients. However, in a similarly designed Japanese randomized phase II trial (JVCG trial), febrile neutropenia (FN) was observed in 34.2% of docetaxel plus ramucirumab arm. This high incidence of FN is a clinical concern when using docetaxel plus ramucirumab for elderly patients. The ASCO practice guideline recommends primary prophylactic granulocyte-colony stimulating factor (G-CSF) when the risk of FN is 20% or higher. PEGylated-G-CSF (pegfilgrastim) administered once a cycle demonstrated reduction of FN incidence in many types of cancers. Based on the above background, we considered that primary prophylactic PEG-G-CSF would be beneficial for elderly NSCLC patients who received docetaxel plus ramucirumab.
a9ded1e5ce5d75814730bb4caaf49419 Method
This is a prospective multicenter, single-arm, phase II study conducted by West Japan Oncology Group (WJOG). Main inclusion criteria includes: chemo-naïve; aged ≥75; histologically or cytologically confirmed NSCLC; ECOG PS 0/1; adequate organ functions; with measurable disease; without contraindication of ramucirumab; written informed consent; and estimated life expectancy of at least 3 months. Intravenous docetaxel (60 mg/m2, day 1) plus ramucirumab (10 mg/kg, day 1) with subcutaneous PEG-G-CSF (3.6 mg, day 2) every 3 weeks is administered until progression. Continuous docetaxel or ramucirumab monotherapy is permitted when intolerable toxicities occur but clinical benefit is obtained by each drug. The primary endpoint is objective response rate (ORR). Secondary endpoints are PFS, OS, disease control rate, and safety. We assumed that the threshold and expected ORR were 20% and 35%, respectively. Based on this, the number of patients was calculated to be 59 to provide a power of 80% with probability of one-sided type I error being 0.05. Taking ineligible patients into account, the sample size was set at 65. When the study results are promising, we plan to conduct a phase III trial to compare docetaxel plus ramucirumab with PEG-G-CSF support vs. docetaxel monotherapy for chemo-naïve elderly NSCLC patients. Clinical trial information: UMIN000030598.
4c3880bb027f159e801041b1021e88e8 Result
Section not applicable
8eea62084ca7e541d918e823422bd82e Conclusion
6f8b794f3246b0c1e1780bb4d4d5dc53
Section not applicable
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-41 - In Vitro Evaluation for Optimal MET-TKI Selection in Lung Cancers with MET Mutations Including Exon 14 Skipping (ID 12825)
16:45 - 18:00 | Author(s): Tetsuya Mitsudomi
- Abstract
Background
MET exon 14 skipping mutation present in 3-5% of adenocarcinoma of the lung is an emerging driver gene alteration. Clinical responses of these tumors to MET-TKI have been reported. However, response rates are not very satisfactory compared with EGFR/ALK/ROS1 TKI. Therefore, it is necessary to create in vitro model system to understand sensitivity/resistance mechanism for various types of MET-TKI, to establish optimal treatment strategy.
a9ded1e5ce5d75814730bb4caaf49419 Method
We introduced MET exon 14 skipping mutation as well as Y1003F, D1010Y which had been also reported to be present in lung cancer, to mouse pro-B cell line, Ba/F3. Since Ba/F3 requires interleukin 3 (IL-3) for its growth, IL-3 independent growth of Ba/F3 indicates that transduced mutation is oncogenic. The growth inhibitory assays were then performed using 9 MET-TKIs that include all classes of MET-TKIs ; Type Ia (crizotinib), Type Ib (capmatinib, tepotinib, savolitinib and AMG337) , Type II (cabozantinib, merestinib and glesatinib) and Type III (tivantinib).
4c3880bb027f159e801041b1021e88e8 Result
Ba/F3 transfected with wild-type MET did not grow in the absence of IL-3, while all transfected with any of three mutated MET did so. In general, all type Ia/b / type II inhibitors were active for any of 3 MET mutations. Interestingly MET point mutations (Y1003F/D1010Y) were more sensitive to type Ib inhibitors except AMG337 than type II, while exon 14 skipping was likely to be more sensitive to type II inhibitors than type Ib compared with point mutations. IC50 / Cmax of cabozontinib was least for exon 14 skipping while that of capmatinib was least for Y1003F and D1010Y, suggesting most promising activity of these drugs (Table).
8eea62084ca7e541d918e823422bd82e Conclusion
We found that the MET exon 14 skipping, Y1003F, and D1010Y mutations were all oncogenic in Ba/F3 system. Several type I/II inhibitors especially cabozantinib and capmatinib are expected to be active for treating lung cancer patients with MET mutations.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.13-42 - Activity of Novel HER2 Inhibitor, Poziotinib, for HER2 Exon 20 Mutations in Lung Cancer and Mechanism of Acquired Resistance (ID 12717)
16:45 - 18:00 | Author(s): Tetsuya Mitsudomi
- Abstract
Background
Oncogenic HER2 mutations are present in 2-4% of adenocarcinoma of the lung. However, clinical trials of HER2 inhibitors such as afatinib or neratinib has been unsatisfactory. Recently, a novel HER2 inhibitor, poziotinib has been developed and clinical trial results are being expected. Here, we evaluated poziotinib in comparison with pre-existing TKIs using Ba/F3 system. We also derived resistant clones against poziotinib and investigated their resistant mechanism.
a9ded1e5ce5d75814730bb4caaf49419 Method
We introduced three common HER2 mutations into Ba/F3 cells (i.e. G776delinsVC (VC), A775_G776insYVMA (YVMA) and P780_Y781insGSP (GSP)) which account for 13, 72, 9% of HER2 mutations in human lung cancer, respectively. We defined sensitivity index (SI) as an IC90divided by trough concentration of a given drug at the recommended dose for humans in the literature, as a surrogate for drug activity in humans. Poziotinib activity was compared with 8 TKIs (afatinib, osimertinib, erlotinib, neratinib, lapatinib, dacomitinib, irbinitinib, and AZ5104). In addition, we created resistant clones by exposing poziotinib in the presence of N-ethyl-N-nitrosourea (ENU) and HER2 secondary mutations were searched.
4c3880bb027f159e801041b1021e88e8 Result
All drugs but lapatinib showed the highest activity against VC (Table). In contrast, YVMA was most resistant in all but neratinib and poziotinib. For most common YVMA, poziotinib was the only drug that had SI of less than 10 (Table). Furthermore, poziotinib was most potent for VC and GSP except dacomitinib for GSP (Table). We established 19 poziotinib-resistant clones, all of which harbored C805S secondary mutation of the HER2 gene homologous to C797S of the EGFR gene.
Ctrough [nM] YVMA VC GSP Afatinib 69 28 5.4 12 Dacomitinib 166 20 4.4 6.6 Erlotinib 2969 337 22 98 Osimertinib 400 40 4.5 30 Neratinib 100 11 11 28 Lapatinib 516 133 117 91 Poziotinib 20 6.0 2.7 10 Irbinitinib 520 34 33 16 AZ5104 50 60 8.0 48
8eea62084ca7e541d918e823422bd82e Conclusion
Poziotinib showed the most potent activity against HER2 exon 20 mutations. We also found that secondary C805S HER2 mutation was the common mechanism of acquired resistance, which most likely inhibit covalent binding of poziotinib with HER2.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.03 - Biology (Not CME Accredited Session) (ID 952)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.03-17 - EGFR T790M Mutation may not be Generated through Selection by EGFR-TKI from Randomly Occurring Mutations in Vitro Using ENU (ID 13205)
16:45 - 18:00 | Author(s): Tetsuya Mitsudomi
- Abstract
Background
It is postulated that T790M resistant mutation is a result of selection by EGFR-TKI from clones that have randomly generated mutations. If so, it would be possible to detect the process of convergence from random mutations to T790M, depending on exposure time for EGFR-TKI.
a9ded1e5ce5d75814730bb4caaf49419 Method
After exposure of Ba/F3 cells expressing EGFR Del19 to ENU (N-ethyl-N-nitrosourea) for 24 hours, they were cultured with various concentrations of 3 EGFR-TKIs at trough concentrations (Fig.). At 1, 6, 12, 24, and 48 hours, cell viability was evaluated and single cell cloning was performed. EGFR exons 18 to 21 were analyzed by Sanger sequencing.
4c3880bb027f159e801041b1021e88e8 Result
Percentages of viable cells exposed to TKI were approximately 80%, 50%, < 20%, and < 3% at 1, 6, 12, 48 hours, respectively. These ratios were almost similar among 3 TKIs. Of 50 clones that had survived TKI treatment, T790M was detected in 12 (24%) (Fig.). Notably, two were detected in cells exposed to erlotinib or afatinib only for one hour (Fig.). In addition, we found 2 G873E (c.2876G>A) mutations, known to be oncogenic, with the same TKI treatment. In contrast, 36 clones (72%) with TKI or additional 19 clones without TKIs that had been exposed to only ENU did not harbor any secondary EGFR mutations.
8eea62084ca7e541d918e823422bd82e Conclusion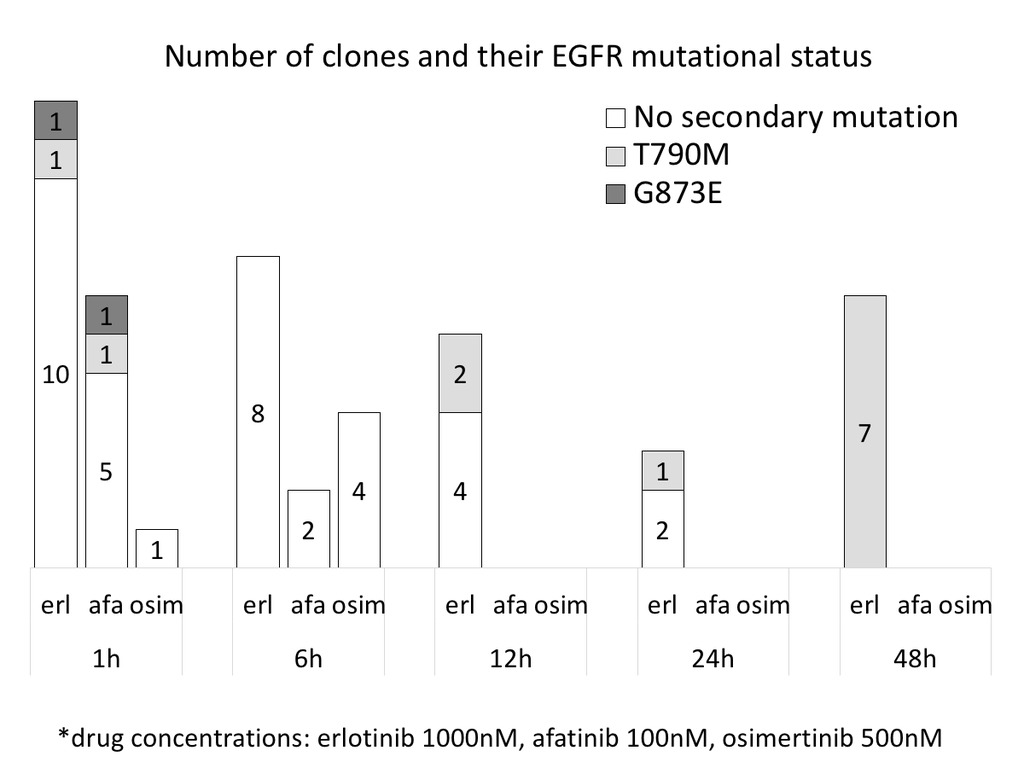
It is unlikely that EGFR T790M mutation is generated through selection by EGFR-TKI from randomly occurring mutations. Rather, it appears that T790 is the preferred target of mutagenesis through yet an unknown mechanism which occurs after only one-hour treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.04 - Immunooncology (Not CME Accredited Session) (ID 953)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.04-15 - Heterogeneity and Correlation Between Immune Markers in Lung Cancers: Analysis of Treatment-Naïve Lesions (ID 11288)
16:45 - 18:00 | Author(s): Tetsuya Mitsudomi
- Abstract
Background
Immunotherapies are becoming a new standard of care for patients with lung cancers. Although a few immune-checkpoints are currently used as therapeutic targets and/or as predictive biomarkers, the complex correlation between immune-checkpoints is not well understood. Expression level of immune-checkpoint molecules is affected by numerous factors including tumor cells themselves, patients’ immunological characteristics, tumor microenvironment (metastatic sites), and previous treatments. To effectively investigate correlations of immune-checkpoints across multiple lesions, we analyzed gene expression data obtained from treatment-naïve autopsied patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
Our cohort of 5 lung cancer patients included thirty specimens of both primary and metastatic lesions. RNA sequencing reads were mapped to the hg19 reference genome using the TopHat/Cufflinks workflow and transcripts were quantified using the FPKM method. Expression data for immune-checkpoints and total numbers of detected mutations were compared.
4c3880bb027f159e801041b1021e88e8 Result
We observed substantial inter-tumor heterogeneity in immune-checkpoint expression between lesions obtained from each patient. No consistent correlation was found by comparison of primary vs. metastatic lesions or between primary vs. specific metastatic sites. Evaluation of immune-checkpoints expressed by tumor cells and/or antigen presenting cells revealed a positive correlation between GAL9 and PD-L2 (R = 0.79) and GAL9 and HVEM (R = 0.69; Figure 1A). We also observed a strong correlation between these markers when lesions obtained from each patient were correlated to each other (Figure 1B and C). Comparisons between immune-checkpoints expressed by immune cells identified a positive correlation between PD-1 and LAG3 (R = 0.77). No correlation was found between immune-checkpoint expression and mutation burden.
8eea62084ca7e541d918e823422bd82e Conclusion
We observed substantial inter-tumor heterogeneity in immune-checkpoints expression in each patient. We also found several positive correlations between immune-checkpoints which were consistent within the small cohort of patients. Further functional evaluation is warranted.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 965)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.16-03 - CheckMate 816: A Phase 3 Trial of Neoadjuvant Nivolumab Plus Ipilimumab or Chemotherapy vs Chemotherapy in Early-Stage NSCLC (ID 12599)
16:45 - 18:00 | Author(s): Tetsuya Mitsudomi
- Abstract
Background
Approximately 20–25% of patients with NSCLC are diagnosed with early or localized disease, which has a relapse rate of 30–80% with surgery. Although neoadjuvant chemotherapy can reduce the risk of relapse, it only provides a pathological complete response (pCR; no viable tumor cells) rate of 4%. The neoadjuvant setting presents abundant tumor-associated neoantigens derived from the primary tumor that may allow immunotherapy to prime a long-lasting immune response. Clinical trial results support the use of immuno-oncology agents as neoadjuvant treatment for early-stage NSCLC. In a pilot study in patients with untreated, surgically resectable early-stage (stage I–IIIA) NSCLC, nivolumab (a fully human PD-1 immune checkpoint inhibitor antibody) administered as neoadjuvant treatment (3 mg/kg for 2 cycles during the 4 weeks prior to surgery) induced a pCR in 10% of patients and a major pathological response (MPR; ≤10% residual viable tumor cells in resected primary tumor) in 45% of patients, did not delay surgery, and was associated with an acceptable safety profile. Combining immuno-oncology agents with distinct mechanisms of action, such as PD-1 and CTLA-4 inhibitors, offers the possibility of a synergistic response and may improve antitumor activity compared with either agent alone. The combination of an immuno-oncology agent and chemotherapy may also offer synergistic activity, given that chemotherapy results in tumor cell death and subsequent antigen release that can activate an immune response. Promising results have been noted with nivolumab plus ipilimumab (a CTLA-4 immune checkpoint inhibitor antibody) and nivolumab plus chemotherapy in patients with treatment-naïve stage IIIB/IV NSCLC in the multicohort phase 1 CheckMate 012 study. CheckMate 816 (NCT02998528) is a phase 3 study evaluating nivolumab plus ipilimumab, nivolumab plus platinum-doublet chemotherapy, and platinum-doublet chemotherapy as neoadjuvant treatment for early-stage NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
Approximately 642 patients aged ≥18 years with early-stage (stages IB–IIIA) resectable NSCLC, ECOG performance status 0–1, pulmonary function capable of tolerating lung resection, and available lung tumor tissue will be enrolled in North America, South America, Europe, Asia, and Africa. Patients are ineligible if they have active autoimmune disease or had received prior treatment with immune checkpoint inhibitors. Patients will be randomized (1:1:1) to receive neoadjuvant nivolumab plus ipilimumab, nivolumab plus platinum-doublet chemotherapy, or platinum-doublet chemotherapy. Primary endpoints are event-free survival and pCR. Key secondary endpoints are overall survival and MPR (<10% residual tumor in lung and lymph nodes). The start date was January 2017. The estimated primary completion date is May 2023.
4c3880bb027f159e801041b1021e88e8 Result
Section not applicable
8eea62084ca7e541d918e823422bd82e Conclusion
Section not applicable
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
PL03 - Call to Action - Challenges Ahead (ID 851)
- Event: WCLC 2018
- Type: Plenary Session
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 08:15 - 09:45, Plenary Hall
-
+
PL03.06 - The Future of Targeted Therapy (ID 11654)
09:30 - 09:45 | Presenting Author(s): Tetsuya Mitsudomi
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.


