Virtual Library
Start Your Search
Kazuhiro Yasufuku
Author of
-
+
MA12 - Mesothelioma Surgery and Novel Targets for Prognosis and Therapy (ID 913)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Mesothelioma
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 10:30 - 12:00, Room 202 BD
-
+
MA12.09 - Preclinical Investigations of Folate Receptor Targeted Nanoparticles for Photodynamic Therapy of Malignant Pleural Mesothelioma (ID 11277)
11:30 - 11:35 | Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Background
Photodynamic therapy (PDT) following lung-sparing extended pleurectomy (EPD) for malignant pleural mesothelioma (MPM) has been investigated as a potential means to kill residual microscopic cells. High expression of folate receptor 1 (FOLR1) has been reported in MPM, and targeting the FOLR1 has been considered as a new potential strategy. We have developed FOLR1-targeting porphyrin-lipid nanoparticles (folate-porphysomes; FP) for PDT. The inhibition of survival pathways of activated epidermal growth factor (EGFR) also enhance the PDT efficacy. Here, we have combined these approaches by using FP based PDT together with an EGFR-tyrosine kinase inhibitor (EGFR-TKI).
a9ded1e5ce5d75814730bb4caaf49419 Method
The frequency of FOLR1 and EGFR expression in MPM was analyzed using tissue microarrays. Confocal microscopy and a cell viability assay were performed to confirm the specificity of FOLR1-targeting cellular uptake and photocytotoxicity in vitro. In vivo fluorescence activation and the therapeutic efficacy were then examined. The effect of EGFR-TKI was assessed in vitro. The in vivo combined anti-tumor effect of EGFR-TKI and FP-PDT was then evaluated.
4c3880bb027f159e801041b1021e88e8 Result
FOLR1 and EGFR were expressed in 79 % and 89 % of the MPM samples, respectively. The intracellular uptake of FP corresponded well with FOLR1 expression. When MPM cells were incubated in FP and then irradiated at 671 nm, there was significant in vitro cell kill, which was inhibited in the presence of free folic acid, suggesting the specificity of FPs. FOLR1 targeting resulted in disassembly of the porphysomes and subsequent fluorescence activation in intrathoracic disseminated MPM tumors, as demonstrated by ex vivo tissue imaging. FP-PDT resulted in significant cellular damage and apoptosis in vivo. Furthermore, the combination of pre-treatment with EGFR-TKI plus FP-PDT showed further marked improvement of treatment responses.
8eea62084ca7e541d918e823422bd82e Conclusion
Folate-porphysome based PDT shows selective destruction of MPM cells based on FOLR1 targeting, and pre-treatment with EGFR-TKI further enhances the therapeutic response.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA13 - Interventional Pulmonology (ID 914)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Interventional Diagnostics/Pulmonology
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 10:30 - 12:00, Room 206 AC
-
+
MA13.05 - The Canada Lymph Node Sonographic Score: National Validation of a Sonographic Score to Determine Mediastinal Lymph Node Malignancy (ID 12084)
11:00 - 11:05 | Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Background
At the time of endobronchial ultrasound (EBUS) staging for Non-Small Cell Lung Cancer (NSCLC), 6 ultrasonic criteria (Fig. 1) are used to assign a Lymph Node Sonographic Score (LNSS) that is predictive of malignancy. The LNSS has not gained widespread use due to lack of research demonstrating its validity and reliability among endoscopists. We hypothesized that LNSS correlates well with the probability of malignancy, potentially guiding decisions for lymph node (LN) biopsy.
a9ded1e5ce5d75814730bb4caaf49419 Method
We conducted a prospective study to assess the validity and reliability of the LNSS. The validation cohort comprised LN that were video-recorded from patients with NSCLC, and assigned a LNSS by an experienced endoscopist. Videos were then circulated to thoracic surgeons and interventional respirologists across Canada, who were asked to assign a score to each LN. All raters had demonstrated proficiency using our online education module, were blinded to staging information, and to each other. Each LN was scored by at least 3 independent raters. Pathological specimens were used as the gold standard for determination of malignancy. Regression, receiver operator curve (ROC), and Gwet’s AC1 analyses were used to test LNSS score performance, discriminatory capacity, and inter-rater reliability.
4c3880bb027f159e801041b1021e88e8 Result
A total of 300 LNs (18% malignant) from 140 patients were analyzed by 11 endoscopists across 7 Canadian centres. LNSS=0 was strongly predictive of benign LN (NPV= 95.69%, OR=49.2, p=0.001). LNSS ≤2.5 (OR=44, p=0.001) was determined as the cutoff for malignancy based on ROC analysis (c= 0.7757, 95%CI: 0.70281-0.84853). Inter-rater reliability for LNSS=0 was 0.8553 (95%CI:0.8158-0.8947, p=0.0001) and 0.46 for LNSS ≤2.5 (95%CI=0.3521-0.5012, p=0.0001).
8eea62084ca7e541d918e823422bd82e Conclusion
The Canada LNSS shows excellent performance in identifying benign LN at the time of EBUS. A cutoff ≤2.5 has the potential to inform decision-making regarding biopsy or repeat biopsy/mediastinoscopy if the initial results are inconclusive. Further teaching and education are required to improve inter-rater reliability.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA22 - New Therapeutics, Pathology, and Brain Metastases for Small Cell and Neuroendocrine Tumour (ID 925)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Small Cell Lung Cancer/NET
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 15:15 - 16:45, Room 206 BD
-
+
MA22.06 - Preinvasive Multifocal Neuroendocrine Lesions with Primary Typical Carcinoid Lung Tumors: A Negative Prognostic Factor? (ID 12432)
15:50 - 15:55 | Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Background
Impact on survival in patients with surgically resected multifocal neuroendocrine lesions (MNET), such as diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) or tumorlets, along with primary typical lung carcinoid (TC) is unclear. Aim of this study is to analyze whether synchronous preinvasive multifocal neuroendocrine lesions of the lung with primary TC tumors (MTNET+TC) may represent a negative prognostic factor.
a9ded1e5ce5d75814730bb4caaf49419 Method
A retrospective study, prospectively collected, for TC from two institutional databases was evaluated with a lifelong follow-up from surgery. Patients who did not receive surgery, underwent bronchial resection or lung transplant were excluded. Pathology specimens were all reclassified according the 2015 WHO and the eight AJCC Staging system. Kaplan-Meier(KM) method and Log-rank test reports significance between TC and were MTNET+TC were used. Hence a 1:1 propensity score matching analyses was done by adjusting the imbalance and comparing the overall survival and progression free rate between matched groups with a Cox proportional hazards regression model. A p value of 0.05 or less was considered significant.
4c3880bb027f159e801041b1021e88e8 Result
From January 1983 to December 2013 a total of 234 patients was outlined from the databases (TABLE). A total of 41 patients (17.5%) with MNET+TC were identified. Overall KM progression free survival achieved at 5 and 10 years respectively MNET+TC 93.2% and 83.8% compare to TC 98.4% and 96.1% (p =0.00039). Thirty-six MNET+TC were matched pairs vs. TC alone. Univariate Cox proportional hazards model for matched patients MNET+TC compared to TC was 2.78 (95% CI=0.84-9.3, p=0.095). Difference in progression free rate between matched groups was p<0.001.
8eea62084ca7e541d918e823422bd82e Conclusion
Synchronous multifocal neuroendocrine preinvasive lesions (MNET) with primary typical carcinoid (TC) lung tumors can be a negative prognostic factor. Careful search of MNET should be always performed in clinical and pathological staging of a suspected primary TC. The increased risk of progression of MNET+TC warrants an accurate and lifelong follow-up.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA23 - Early Stage Lung Cancer: Present and Future (ID 926)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment of Early Stage/Localized Disease
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 105
-
+
MA23.10 - Cone-Beam Computed Tomography-Guided Microcoil Localization of Pulmonary Nodules During Video-Assisted Thoracic Surgery (ID 13345)
11:35 - 11:40 | Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Background
The standard procedure at our institution for intraoperative localization of non-palpable small lung nodules is computed tomography (CT)-guided microcoil placement prior to video-assisted thoracic surgery (VATS). Typically, microcoil placement is performed in the radiology suite followed by transfer to the operation room (OR). Our institution has built the Guided Therapeutics (GTx) OR, which includes a robotic cone-beam CT (CBCT). The GTx OR allows imaging and therapy to occur in one location. This can improve workflow and reduce patient transportation, which may increase the risk for microcoil dislodgement or the development of pneumothorax/hemothorax. Our objective was to determine the safety and efficacy of CBCT-guided microcoil placement for nodule localization during VATS.
a9ded1e5ce5d75814730bb4caaf49419 Method
This is a single center phase I clinical trial (NCT02496624). Patients with small lung nodules who were candidates for standard CT-guided microcoil localization were enrolled. CBCT was used to generate a 3D reconstruction. The lesion was then segmented using Syngo iGuide software. This reconstruction was next integrated into the digital workspace and automatically registered onto the fluoroscopic images, creating ‘augmented fluoroscopy’. The microcoil was placed percutaneously using ‘augmented’ guidance, proximal to the lesion, using local anesthetic. Patients were subsequently induced into general anesthesia, intubated, and positioned for VATS. Minimally invasive resection of the nodule together with the microcoil was performed under standard fluoroscopic guidance.
4c3880bb027f159e801041b1021e88e8 Result
A total of 11 patients were enrolled (mean age 70 ± 11SD). The average tumor size on CT was 1.3 cm (range 0.9-1.7). The average deepest depth from the pleural surface was 2.3 cm (1.3-3.8). The average CBCT-guided intervention time was 39 minutes (25-54), and VATS procedural time was 54 minutes (14-78). We were able to detect and successfully resect all nodules. Average total radiation dose was in an acceptable low range (8307 μGy*m2, range, 2402–18,371). There were no intraoperative complications. Average post-operative length of stay was 1.8 days. A pathological diagnosis was made for all patients: 8 primary lung cancers and 3 lung metastases. All surgical margins were negative on final pathology.
8eea62084ca7e541d918e823422bd82e Conclusion
CBCT-guided microcoil insertion followed by VATS was safe, with short operative times, short length of stay and 100% diagnostic yield. With the GTx OR’s real-time guidance capabilities, surgeons can operate with increased confidence of finding and removing the target lesion. This technique will become increasingly important in the future with growing numbers of small nodules being detected on CT by lung cancer screening programs.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA27 - Novel Drugs and PDX Models (ID 931)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 13:30 - 15:00, Room 206 BD
-
+
MA27.01 - Establishment of PDX From Tumors Characterized by EGFR Mutations or ALK Fusion Genes from Resections, Biopsies and Pleural Fluids (ID 12144)
13:30 - 13:35 | Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Background
Patient-derived xenograft (PDX) models allow for cancer tissue expansion, providing an effective method to evaluate tumor biology and mechanisms of response or resistance. Our study aims to establish models in patients enriched for lung adenocarcinoma (LUAD) with EGFR mutations or ALK fusion genes which respond initially to oral targeted therapy, but typically develop resistance and disease relapse within 2 years. The PDXs will be evaluated for their potential to model therapy outcomes, to determine resistance mechanisms and to evaluate novel therapy strategies to overcome resistance.
a9ded1e5ce5d75814730bb4caaf49419 Method
From August 2015 to January 2018, we collected 109 samples from patients with EGFR- or ALK-driven LUAD and from never-smoker LUAD patients with unknown mutation status. Five samples with low tissue viability (i.e. necrotic) or very low tumor content (<100 malignant cells) were excluded. Adequate samples were implanted into the subcutaneous tissue of NOD-SCID mice. At this time, 16 samples have reached the study endpoint (tumor growth ≥1.5cm3) and 60 showed no tumor-growth following implantation (median follow-up: 8m). Results are currently pending for 18 models.
4c3880bb027f159e801041b1021e88e8 Result
Samples were collected from surgical resections (31, 36%), CT-guided biopsies (12, 14%), EBUS (19, 22%) and pleural fluid effusions (24, 28%). Most patients were female (51/86, 59%), never smokers (62/85, 73%), and had stage III or IV cancer (55/79, 70%). Mutations in EGFR and ALK were found in 55/81 (68%) and 12/84 (14%) primary cancers, respectively. Early-passage xenograft engraftment (XG) was observed in only 16 (19%) PDXs, including 9/55 (16%) EGFR- and 1/12 (8%) ALK-mutant cancers. The phenotype and molecular changes (EGFR and ALK) were consistent within the PDX model and its corresponding patient sample. Samples collected from surgical-resection specimens showed a trend towards higher engraftment rates (p=0.084). Conversely, the presence of EGFR or ALK mutations showed a trend towards non-engraftment (noXG, p=0.075). Patient smoking status and tumor stage did not influence engraftment rate. To identify reasons for no tumor-growth, we conducted histological analysis in the subcutaneous fat-pads (nodes in the implant sites) of 28 noXG mice. Interestingly, we identified small non-palpable foci of carcinoma in 8 animals (4 EGFR+ and 2 ALK+).
8eea62084ca7e541d918e823422bd82e Conclusion
Environmental or molecular factors may impair engraftment rates of EGFR+ and ALK+ LUAD samples in PDX models. Nevertheless, these models recapitulate the primary disease and could be useful for population-based drug-screening studies.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MS09 - Tumour Board - Tissue Acquisition and Staging (ID 788)
- Event: WCLC 2018
- Type: Mini Symposium
- Track: Interventional Diagnostics/Pulmonology
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 15:15 - 16:45, Room 206 BD
-
+
MS09.01 - Cases Prepared by Drs. Edell/Murgu (ID 11436)
15:15 - 16:35 | Presenting Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-76 - The Impact of Concordance with a Lung Cancer Diagnosis Pathway Guideline on Treatment Access in Patients with Stage IV Lung Cancer (ID 12628)
16:45 - 18:00 | Author(s): Kazuhiro Yasufuku
- Abstract
Background
Lung cancer is the leading cause of cancer mortality with the majority of cases diagnosed at an advanced stage. Timely access to treatment is dependent on efficient and appropriate patient assessment and early referral for diagnostic workup. This study aims to assess the impact of referral concordance with a new Lung Cancer Diagnostic Pathway Guideline (LCDPG) on access to treatment in patients with stage IV lung cancer.
a9ded1e5ce5d75814730bb4caaf49419 Method
This is a retrospective cohort study of patients with clinical stage IV lung cancer referred to the Diagnostic Assessment Program (DAP) at a Canadian tertiary cancer centre between November 1, 2015 and May 31, 2017. Patient referrals were defined as concordant or discordant based on Cancer Care Ontario LCDPG. The primary outcome; time to treatment from initial healthcare presentation; was compared between the concordant and discordant referrals.
4c3880bb027f159e801041b1021e88e8 Result
Two hundred patients were referred for clinical stage IV lung cancer during the study period. Of these referrals, 151 (75.5%) were assessed and referred in concordance with LCDP guidelines. Guideline concordant referrals were associated with reduced time to treatment from first healthcare presentation compared with guideline discordant referrals (55.3 vs 108.8 days, p<0.001). Time to diagnostic procedure (32.2 vs 86.7 days, p<0.001) and decision to treat (38.5 vs 93.8 days, p<0.001) was also reduced with guideline concordance. The most common reason for discordant assessment and referral was delayed or inadequate investigation of symptoms in a high risk patient (32.7% of discordant referrals).
The mean time from referral to diagnostic procedure (19.4 [SD 16.0] days), decision to treat (23.3 [SD 17.1] days), and treatment initiation (39.7 [SD 26.3] days) did not significantly differ between concordant and discordant groups. Time from referral to decision to treat was within 28 days in 71.5% of patients. The mean number of hospital visits from referral to treatment was 4.9 (SD 3.5). Diagnosis was achieved with a single diagnostic test in the majority of patients (91%). The most common method of diagnosis was EBUS-TBNA (33.5%). The most common treatment modalities initiated were radiation (60.5%) followed by chemotherapy (43%) and targeted therapy (21.5%).
8eea62084ca7e541d918e823422bd82e Conclusion
Guideline concordant assessment and referral of patients with stage IV lung cancer results in reduced time to diagnosis and treatment. The utilization of a LCDPG for lung cancer provides a streamlined and efficient framework to facilitate early diagnosis and treatment. Future research and education should focus on improving factors leading to a delay in DAP referral.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.09 - Pathology (Not CME Accredited Session) (ID 975)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.09-14 - Pathobiology of Notch2 in Lung Cancer (ID 13789)
12:00 - 13:30 | Author(s): Kazuhiro Yasufuku
- Abstract
Background
Notch signaling is known to be involved in the initiation, progression, and suppression of various types of cancers. The pathological significance of Notch1 has been well studied in lung cancer, but that of Notch2 is still unclear.
a9ded1e5ce5d75814730bb4caaf49419 Method
Immunohistochemical study was performed to measure the expression of NOTCH2 in non-neoplastic lung tissues and lung cancers in comparison with the Clara (Club) cell 10 kD protein (CC10), and Western blotting analysis was performed to detect NOTCH2 in human cancer cell lines. Notch2 gene knockdown experiment and transient transfection of the intracellular domain of the Notch2 (N2ICD) gene were conducted to reveal the function of Notch2. In addition, we studied the relationships between the expressions of Notch1, 2, and 3.
4c3880bb027f159e801041b1021e88e8 Result
Immunohistochemical study of lung tissues revealed that NOTCH2 was detected in bronchiolar epithelial cells and was often colocalized with CC10, and that adenocarcinoma tissues were more positively stained than those of squamous cell carcinoma and small cell carcinoma tissues. In human lung cancer cell lines the expression of NOTCH2 was similar to that of NOTCH1 and preferentially detected in non-small cell lung carcinoma (NSCLC) cell lines. Knockdown experiments of the Notch2 gene in NSCLC cell lines showed no significant changes in the expression of molecules associated with cell differentiation, proliferation, apoptosis, and motility. The effects of Notch2 gene knockdown could have be masked by concomitant Notch1 activation, as indicated by an increase in the intracellular domain of NOTCH1. Additionally, the transient transfection of the N2ICD gene induced CC10 expression in an adenocarcinoma cell line.
8eea62084ca7e541d918e823422bd82e Conclusion
6f8b794f3246b0c1e1780bb4d4d5dc53
The present study revealed that Notch2 is important in Club cell differentiation in normal lungs and in adenocarcinoma. We also determined that Notch1 and Notch2 are covariant, and the balance of the expression of Notch receptors could determine the biological behaviors of lung cancer cells.
-
+
P3.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 982)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.16-05 - A Nanotechnology-Enabled Strategy for Image-Guided Transbronchial and Transpleural Photothermal Therapy of Peripheral Lung Cancer (ID 12048)
12:00 - 13:30 | Author(s): Kazuhiro Yasufuku
- Abstract
Background
Surgical resection has been established as standard of care for early-stage peripheral non-small cell lung cancer (NSCLC). However, the development of non-surgical treatment and minimally invasive therapeutics is urgently needed due to the known morbidity of surgery, particularly in high-risk patients. We have previously demonstrated the potential of porphysome nanoparticle-enabled fluorescence-guided photothermal therapy (PTT) of peripheral lung cancer in preclinical animal models. In an effort to prepare this technology for clinical application, we developed a porphysome-specific fiberscope (scanning fiber endoscope [SFE]) and porphysome-specific thoracoscope (P-PINPOINT), both capable of operating in fluorescence mode, for image-guided transbronchial and transpleural PTT to treat endo-/peribronchial and subpleural tumors respectively.
a9ded1e5ce5d75814730bb4caaf49419 Method
In our study, we used three animal models: an in vivo human lung cancer xenograft (A549) in mice, another in vivo orthotopic VX2 tumor in rabbits, and an ex vivo pig lung into which A549 tumor tissue was transplanted. Forty-eight hours after intravenous injection of the porphysomes, the animals were used for imaging evaluation by SFE and P-PINPOINT, followed by photothermal ablation.
4c3880bb027f159e801041b1021e88e8 Result
The SFE, whose 1.2mm diameter is small enough to pass through the working channel of a conventional bronchoscope, could visualize porphysome-laden tumors located inside or close to the peripheral bronchial wall. The P-PINPOINT system had high sensitivity for porphysome fluorescence and enabled image-guided thoracoscopic resection of porphysome-accumulated tumors close to pleural. Porphysomes also enhanced the efficacy of SFE-guided transbronchial PTT and P-PINPOINT-guided transpleural PTT, resulting in selective and efficient tumor tissue ablation in the rabbit and pig models.
8eea62084ca7e541d918e823422bd82e Conclusion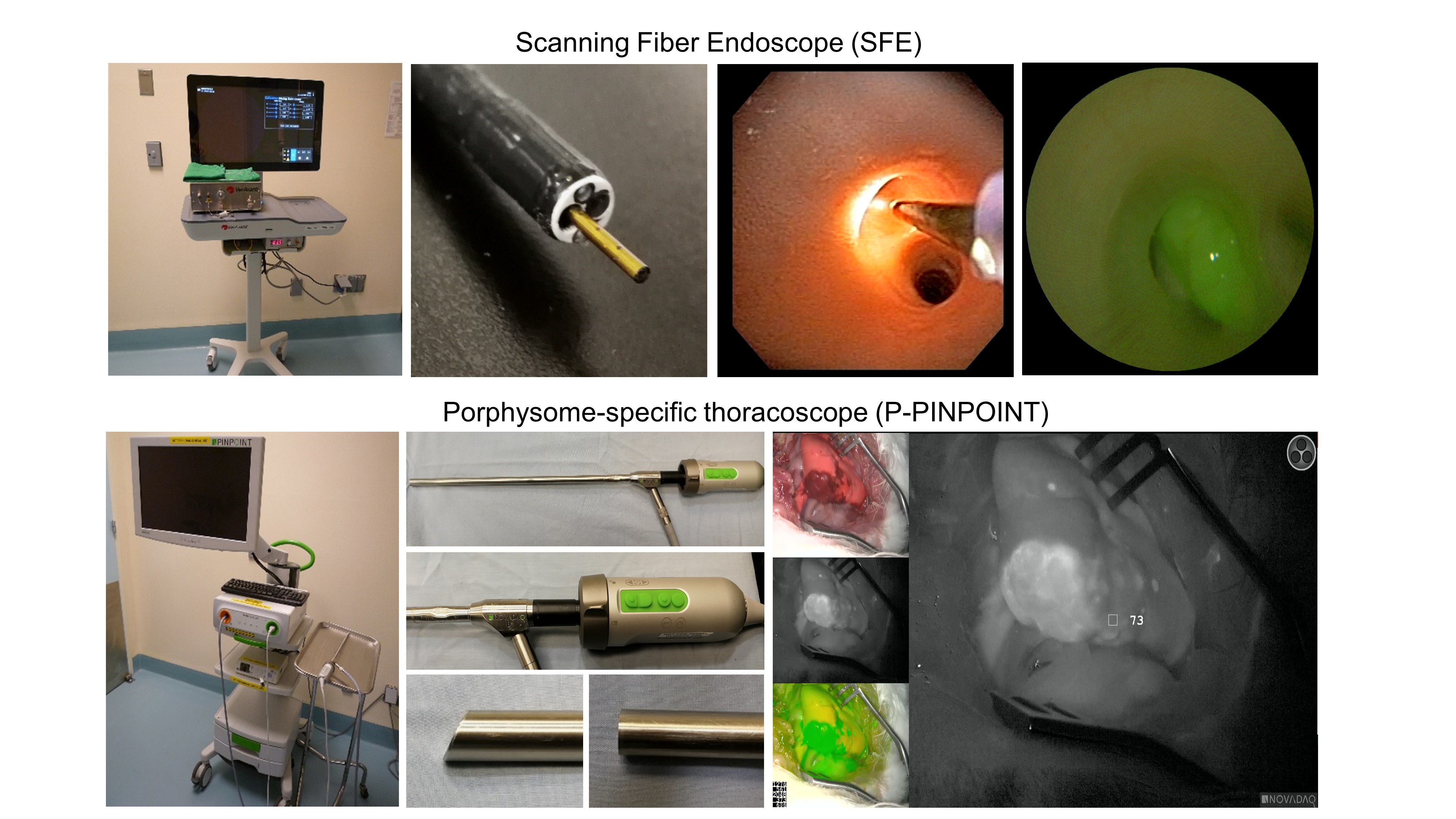
These promising results suggest potential in the clinical translation of this novel platform to impact non-surgical and minimally invasive treatment options for early-stage peripheral lung cancer. This could offer new strategies for the treatment of non-small cell lung cancer.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
WS03 - Endoscopic Diagnosis and Staging of Lung Cancer – Interventional Pulmonology Hands-On Workshop (Ticketed Session) (ID 986)
- Event: WCLC 2018
- Type: Workshop
- Track: Interventional Diagnostics/Pulmonology
- Presentations: 1
- Moderators:
- Coordinates: 9/23/2018, 08:00 - 11:30, Room 205 BD
-
+
WS03.02 - EBUS-TBNA – Role in Invasive Mediastinal Staging (ID 14658)
08:10 - 08:30 | Presenting Author(s): Kazuhiro Yasufuku
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.


