Virtual Library
Start Your Search
Kim Lori Sandler
Author of
-
+
P1.11 - Screening and Early Detection (ID 177)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Screening and Early Detection
- Presentations: 1
- Now Available
- Moderators:
- Coordinates: 9/08/2019, 09:45 - 18:00, Exhibit Hall
-
+
P1.11-31 - Understanding Referral Patterns in a Lung Cancer Screening Program (Now Available) (ID 726)
09:45 - 18:00 | Author(s): Kim Lori Sandler
- Abstract
Background
Lung cancer screening (LCS) provides an important way to reduce the morbidity and mortality of lung cancer, however, the uptake of LCS has remained strikingly low, which has been attributed to both patient and provider level factors. We examined referring clinician and patient characteristics to direct interventions to promote LCS.
Method
We examined the sociodemographic and geophysical characteristics of patients and their initial referring providers at a single institution LCS program from 1/1/2015-7/15/2018. Using data from the clinical record, we geocoded patients, generated maps, and estimated area deprivation indices, a proportional estimate of community socioeconomic status incorporating education, employment and poverty, at the census track level. We performed descriptive analysis of patient level factors such as race, insurance status, and their distribution across referring providers.
Result
1085 unique individuals—909 (84.0%) whites, 143 (13.2%) blacks, and 30 others (3.7%) were referred for LCS by 268 unique providers with 36 cancers detected. Approximately half (137) of providers referred one only individual and 27(10.1%) referred 52% of the patients screened. Primary care providers consisted of 76.4% of referrers, followed by pulmonologists (7.6%) and oncologists (5.3%). 211 referrers (78.7%) were affiliated with our institution. Referral of non-white patients was uniformly distributed among 170 referring providers. Referrals were geographically clustered in counties neighboring the hospital (Figure 1). Average area deprivation was 0.347 with a broad range (0.0770-0.838), with no difference between whites and blacks (0.345 vs 0.354, p=0.482), despite higher PLCO model estimated risk in blacks(7.1 vs 4.9, p<0.001).
Conclusion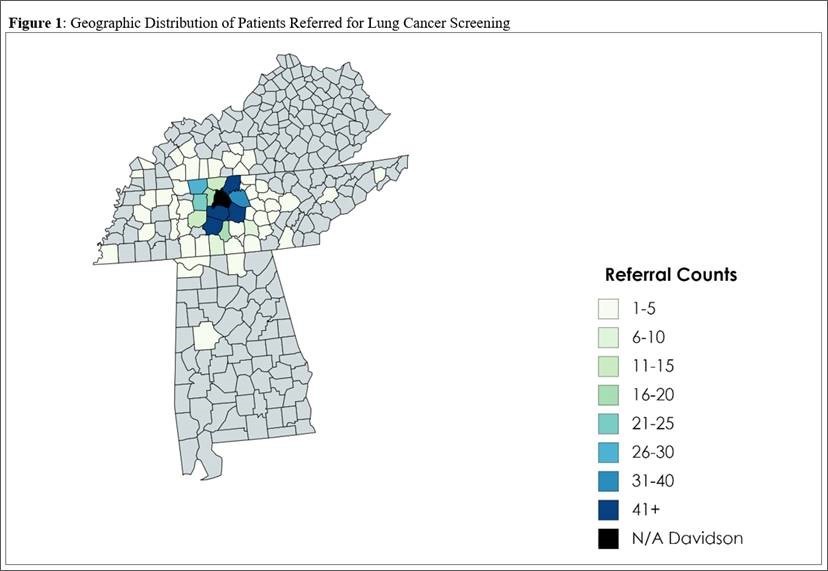
A small number of physicians referred the majority of patients for LCS, however the distribution of minorities was nearly uniform across referrers. We demonstrate broad knowledge of LCS by the large number of referrers, in spite of low referral volume by most providers. These results are guiding ongoing initiatives to improve the overall uptake of LCS by targeted provider communication and community education strategies.
-
+
P2.11 - Screening and Early Detection (ID 178)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Screening and Early Detection
- Presentations: 1
- Moderators:
- Coordinates: 9/09/2019, 10:15 - 18:15, Exhibit Hall
-
+
P2.11-01 - Novel Flexible Longitudinal Machine Learning Coupled with Patient Demographics Improves Lung Cancer Risk Prediction Using Whole Screening CTs (ID 2856)
10:15 - 18:15 | Presenting Author(s): Kim Lori Sandler
- Abstract
Background
The inherent variability of screening scans in clinical practice limits existing machine and deep learning techniques, who have difficulty accessing all available information. As a result, cohorts are less generalizable or require integrating human intervention to initiate data definition. We created a deep learning prediction model for lung cancer screening that could define temporally static and time varying imaging features and combine them with common epidemiological factors collected at the time of shared decision making. We propose a new longitudinal lung cancer detection method, called longitudinal imaging and clinical data co-learning (LICDC), which integrates the temporally flexible deep imaging features and clinical features from the PLCO model.
Method
722 individuals with cancer and 1072 random participants without cancer with more than one scan were selected from the NLST. We initiated the deep learning model using Kaggle contest algorithm discovered features for cancer using each CT scan (n=4781) in its entirety as an independent observation (temporally varying features are not created). We then applied our flexible long and short-term temporal memory methodology to extract additional longitudinal features and generate a neural network model. This training for the LICDC model used NLST participants (n=829) and scans (n=3588) and 826 participants (367 had multiple scans) with 1193 scans from our local screening program. Ten-fold cross-validation was performed on a participant basis. Cancer risk probabilities from imaging-only deep learning model (DLSTM) were combined with PLCO model predictions and regressed together (LICDC-Logistic Regression). Additionally, individual variables from the PLCO model and the cancer probability from DLSTM were fitted with support vector machine (SVM) method using linear kernels (LICDC-SVM) and their area under the receiver operating curve was estimated (AUC). All AUC curves were calculated from the same 1655 patients (829 from NLST and 826 from our local screening program).
Result
PLCO’s predicted risk and basic imaging features (Kaggle winner) had similar accuracy with AUC of 0.815 and 0.781, respectively. Combining risk estimates from the full longitudinal deep learning and PLCO model in a simple logistic regression increased AUC to 0.861. Re-estimation of PLCO and imaging features LICDC-SVM further increased AUC to 0.918.
Conclusion
 Risk prediction for lung cancer in patients who are eligible for screening is improved by combining longitudinal machine learning CT imaging data with demographic information. These methods allow for risk prediction based on a complete CT imaging data set with varying time between scans and across participants without relying on a segmented nodule.
Risk prediction for lung cancer in patients who are eligible for screening is improved by combining longitudinal machine learning CT imaging data with demographic information. These methods allow for risk prediction based on a complete CT imaging data set with varying time between scans and across participants without relying on a segmented nodule.


