Virtual Library
Start Your Search
Suna Zhou
Author of
-
+
OA06 - Refining Lung Cancer Screening (ID 131)
- Event: WCLC 2019
- Type: Oral Session
- Track: Screening and Early Detection
- Presentations: 1
- Now Available
- Moderators:Tomasz Grodzki, Lluis Esteban Tejero
- Coordinates: 9/09/2019, 11:00 - 12:30, Hilton Head (1978)
-
+
OA06.07 - Discrimination of Lung Invasive Adenocarcinoma with Micropapillary Pattern Based on CT Radiomics (Now Available) (ID 399)
11:00 - 12:30 | Author(s): Suna Zhou
- Abstract
- Presentation
Background
To develop and validate the radiomics nomogram on the discrimination of lung invasive adenocarcinoma (IAC) with micropapillary pattern from non-micropapillary pattern lesion and improve the diagnostic accuracy rate of lung invasive adenocarcinoma with micropapillary pattern before operations and provide guidance for follow-up treatments.
Method
Forty-one pathologically confirmed lung invasive adenocarcinomas with micropapillary pattern from January 2014 to December 2018 were included. Eighty-two pathologically confirmed lung invasive adenocarcinomas without micropapillary pattern from January 2018 to December 2018 were collected. Select 86 patients (70%) randomly from the 123 patients as the primary cohort, and the other 37 patients (30%) were set as an independent validation cohort. Least absolute shrinkage and selection operator (Lasso) was used for feature selection based on contrast enhancement CT images and then radiomics signature building. ROC analysis and AUC were used to value the ability to identify the lung invasive adenocarcinomas with micropapillary pattern.
Result
According to GrayLevelCooccurenceMatrix3, Intensity Histogram and Shape, nine hundred and eighty-five radiomics features were extracted by IBEX. And after data pre-processing such as eliminating missing items, strong correlation variables and multicollinear variables, the features were reduced to 40 features. Based on Mann-Whitney U Test, 28 features were figured out from the 40 features. Then Lasso was used to reduce the features to 3 features (10-1clusterprominenc, -333-4clusterprominence, 8-1contrast) as the most meaningful discriminators to build the radiomics signatures (Table 1). According to SPSS21.0 binary logistic regression analysis, ROC analysis and AUC show that the radiomics signature have effective discrimination performance of lung invasive adenocarcinoma with micropapillary pattern from non- micropapillary pattern lesion (AUC=0.766) and it reflects better in the independent validation cohort (AUC=0.807) (Figure 1).
Table 1 Three characteristic prediction parameters in radiomics label
prediction parameter P value U value W value AUC 10-1clusterprominence <0.005 765.000 4168.000 0.772 -333-4clusterpromise <0.005 790.000 4193.000 0.765 8-1contrast <0.005 919.000 4322.000 0.727
Conclusion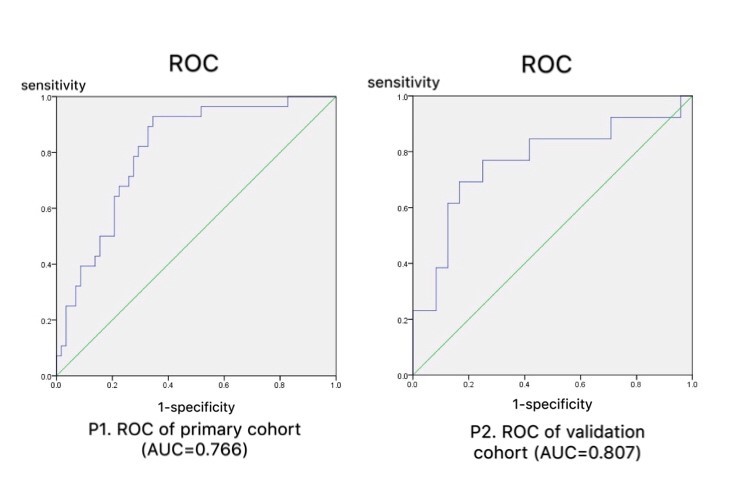
The radiomics signature established in this study have effective prediction of lung invasive adenocarcinoma with micropapillary pattern and non- micropapillary pattern lesion.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.03 - Biology (ID 161)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Biology
- Presentations: 2
- Now Available
- Moderators:
- Coordinates: 9/08/2019, 09:45 - 18:00, Exhibit Hall
-
+
P1.03-17 - Function of Antisense LncRNA RP11-539E17.5 and FAM83A-AS1 Up-Regulating FAM83A in Lung Adenocarcinoma Tumorigenesis and Development (Now Available) (ID 502)
09:45 - 18:00 | Author(s): Suna Zhou
- Abstract
Background
Chromosome 8q24 lies in a gene desert with only a few predicted coding genes, but its variation is associated with multiple tumors. FAM83A in this region activates both PI3K/AKT/TOR and RAS/MAPK signaling cascades downstream of EGFR. The contribution of long noncoding gene in the antisense chain of FAM83A (RP11-539E17.5 and FAM83A-AS1) to the risk of lung adenocarcinoma (LUAD) need to be studied.
Method
We use systematic informatics method to identify the differential expression genes of the Cancer Genome Atlas (TCGA) LUAD transcriptome sequencing data. Then use data mining method to analysis the lncATLAS, USCS, Cistrome, and ENCODE database to infer the biological role of these two lncRNAs. Significant analysis of gene ontology (GO) terms enrichment analyzed the co-expression genes of RP11-539E17.5 in the LUAD and adjacent tissues. At last we use cell experiment to verify the lncRNA function.
Result
FAM83A-AS1 and RP11-539E17.5 located in Chromosome 8q24 were significantly different expressed between cancer and adjacent tissues (log2foldchange>5, p<0.05). The two lncRNA expression levels had notable correlation with FAM83A expression (coR> 0.8, P<0.005) and were significantly associated with patient overall survival and clinical stage (p<0.05). RP11-539E17.5, located in the enhancer element GH08J123181,belong to the enhancer lncRNA group. lncRNA subcellular localization data (lncATLAS) results showed that RP11-539E17.5 transcripts in K562 cells is mainly distributed in the nucleus, and most around the nucleolus and chromosomes. USCS, Cistrome, and ENCODE data mining identified four transcription factors (FOSL2, CTBP2, CEBPB, and MAFK) have significant correlation with the expression of FAM83A-AS1 and RP11-539E17.5. In K562 cell line with high expression of FAM83A-AS1, RP11-539E17.5 and FAM83A showed that expanded regions of RP11-539E17.5 gene sequence were marked by enhancer-specific modifications (H3K27ac domains and H3K4me3 domains). This region also showed an excellent source of DNase sensitive site, and with several binding sites for CTCF, FOSL2, CTBP2, CEBPB and MAFK. In addition, RP11-539E17.5 gene also with enriched DNA methylation feature. RP11-539E17.5 and FAM83A-AS1 knocked down both inhibited the expression of FAM83A the proliferation of PC9, and RP11-539E17.5 gene owning a more significant effect. GO terms enrichment analysis of the co-expressed genes with RP11-539E17.5 showed that the most significantly activated biological pathways is involved with Cell cycle,and cell mitotic signaling pathway, while the molecular function involved with transcription factor binding, DNA binding, ATP binding, endonuclease activity, DNA topoisomerase activity.
Conclusion
We found a LncRNA RP11-539E17.5 with enhancer character up regulate FAM83A expression in LUAD. These results can lay a theoretical foundation for LUAD corresponding countermeasures.
-
+
P1.03-34 - The lncRNA NEAT1 Promotes Radioresistance via the MiR-491-5p/CAPG Axis in NSCLC (Now Available) (ID 89)
09:45 - 18:00 | Presenting Author(s): Suna Zhou
- Abstract
Background
Long noncoding RNAs (lncRNAs) have been implicated in various biological processes and pathological conditions in cancer. However, the exact roles of LncRNA NEAT1 and its underlying mechanisms in radioresistance of non-small cell lung cancer (NSCLC) remain largely unclear.
Method
The expression of LncRNA NEAT1 was measured in NSCLC tissues and cell lines by reverse transcription‑quantitative polymerase chain reaction (RT-qPCR). The radiosensitivity of NSCLC cells including H358, H226, HCC827, H1975, H1395 and H23 were detected by colony formation. Lentivirus-mediated short hairpin RNAs were used to knock down NEAT1 expression in H358 cells. Furthermore, the role of NEAT1 on tumor cell biological behavior and radioresistance were explored through MTT, colony formation, transwell migration, and invasion assays in vitro. Luciferase reporter assay was used to verify interaction between miR-491-5p and NEAT1, CAPG. The potential mechanism of LncRNA NEAT1 was identified by Western blot. Additionally, the association between the survival time and miR-491-5p expression in lung adenocarcinoma patients were evaluated based on the TCGA data.
Result
NEAT1 was highly expressed in NSCLC tissues and cell lines. NEAT1 was up-expressed in radiosensitive NSCLC cells and low-expressed in radioresistant NSCLC cells. Conversely, miR-491-5P was low-expressed in radiosensitive NSCLC cells and up-expressed in radioresistant NSCLC cells. In addition, we revealed a reciprocal repression between NEAT1 and miR-491-5P. CAPG was identified as a down-stream target of miR-491-5P. Further experiments revealed that lncRNA NEAT1 silencing inhibited cell proliferation, invasion and radioresistance in vitro. Overexpression of CAPG rescued the effects of NEAT1 downregulation on proliferation, invasion and radioresistance. In addition, mechanistic analysis showed that lncRNA NEAT1 upregulated the miR-491-5p-targeted gene CAPG through acting as a competitive “sponge” of miR-491-5p. By cox regression analysis, a tendency towards a survival benefit in patients with high miR-491 expression was observed in 430 lung adenocarcinoma patients of the TCGA database.
Conclusion
Our findings suggest that NEAT1 regulated proliferation, invasion and radioresistance by modulating the miR-491-5p/CAPG axis in NSCLC.
-
+
P1.18 - Treatment of Locoregional Disease - NSCLC (ID 190)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Treatment of Locoregional Disease - NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/08/2019, 09:45 - 18:00, Exhibit Hall
-
+
P1.18-15 - Dosimetric and Toxicity Benefits of Adaptive IMRT in Patients with Stage III Non-Small Cell Lung Cancer (ID 396)
09:45 - 18:00 | Author(s): Suna Zhou
- Abstract
Background
Multiple studies observed anatomical changes or tumor shrinkage during concurrent chemoradiotherapy in patients with non-small cell lung cancer (NSCLC). Mid-treatment CT based adaptive radiotherapy targeting to the shrunken tumor can reduce the dose to adjacent normal tissue or potentially deliver a higher dose to the tumor. We aimed to quantitatively analyze the benefit of intensity-modulated radiotherapy (IMRT) adapting to CT changes at the 20th fraction in stage III NSCLC patients.
Method
We retrospectively evaluated consecutive patients with unresectable stage III NSCLC treated with adaptive IMRT from November 2017 to August 2018. The eligibility criteria included a mid-treatment CT simulation for replanning at the 20th fraction and a follow-up of at least 6 months. The prescribed dose was 64-66 Gy in 30 fractions unless exceeding the dose limit. Normal tissues were delineated according to RTOG1106 atlas on organs at risk under the supervision of a senior physician. Dose-volume histograms were calculated for the initial plans, composite adaptive plans, and lung isotoxic boost plans. Radiation pneumonitis (RP) and esophagitis (RE) were graded per CTCAE v4.03. Univariate logistic regression was applied to analyze the correlation between dosimetric factors and adverse events.
Result
53 patients were eligible in this study. The average GTV shrinkage was -40.9% at the 20th fraction. Comparing the dosimetric factors of the composite adaptive plans to the initial ones, the GTV coverage was found marginally higher (P=0.002). The doses to normal tissues were significantly lower (all Ps<0.001) in heart mean dose by 109.5 cGy, esophagus V60 by 1.53%, cord maximum dose by -272.7 cGy, lung V20 and mean lung dose (MLD) by 1.11% and 79.2 cGy, respectively. The tumor targets could potentially get an average lung isotoxic boost of 481 cGy. Eight patients (15.1%) had grade 2 RP while no grade 3 or higher RP occurred. Twenty-three patients (43.4%) developed grade ≥ 2 RE. MLD was significantly associated with grade 2 RP with an odds ratio of 1.39 per 100 cGy increase (95% CI, 1.01 to 1.91; P=0.042). Esophagus V60 was significantly associated with grade ≥ 2 RE with an odds ratio of 1.15 per 1% increase (95% CI, 1.04 to 1.28; P=0.009). (Table 1)
ConclusionFactors
Initial Plans
Adaptive Plans
Mean difference
95%CI
P Value
Targets
PGTV (%)
92.96
93.81
0.85
0.33
1.37
0.002
PTV(%)
94.13
94.54
0.41
0.35
0.80
0.033
Lung
V5(%)
46.77
45.72
-1.05
-0.70
-1.41
<0.001
V20(%)
25.15
24.04
-1.11
-0.80
-1.42
<0.001
V30(%)
18.62
17.60
-1.02
-0.77
-1.27
<0.001
MLD (cGy)
1411.4
1332.2
-79.2
-60.1
-98.4
<0.001
Heart
V30(%)
17.40
15.11
-2.29
-0.94
-3.62
0.001
V40(%)
10.87
9.06
-1.81
-0.98
-2.64
<0.001
V55(%)
4.06
2.79
-1.27
-0.76
-1.77
<0.001
Mean Dose(cGy)
1504.5
1395.0
-109.5
-67.88
-151.22
<0.001
Pericardium
V30(%)
32.17
30.43
-1.74
-0.77
-2.70
0.001
V40(%)
25.70
24.00
-1.7
-0.76
-2.64
0.001
V55(%)
13.87
11.87
-2
-1.34
-2.66
<0.001
Mean Dose(cGy)
2192.9
2091.2
-101.7
-60.00
-143.3
<0.001
Esophagus
V40(%)
39.43
36.49
-2.94
-1.62
-4.27
<0.001
V50(%)
27.89
24.08
-3.81
-2.36
-5.27
<0.001
V60(%)
7.57
6.04
-1.53
-0.96
-2.09
<0.001
Max Dose(cGy)
6498.3
6336.7
-161.6
-101.99
-221.3
<0.001
Cord
Max Dose(cGy)
4113.0
3840.3
-272.7
-209.51
-335.93
<0.001
By adapting to the changes on CT scans at the 20th fraction, the adaptive IMRT approach provides significant dosimetric benefits and has the potential to lower the risk of symptomatic pneumonitis and esophagitis in stage III NSCLC.
-
+
P2.03 - Biology (ID 162)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Biology
- Presentations: 1
- Moderators:
- Coordinates: 9/09/2019, 10:15 - 18:15, Exhibit Hall
-
+
P2.03-05 - PHLPP1 Expression Through AKT and ERK Dual Signaling Pathways May Slow Down the Resistance to TKI in EGFR-Mutated Lung Adenocarcinoma (ID 298)
10:15 - 18:15 | Author(s): Suna Zhou
- Abstract
Background
The epidermal growth factor receptor (EGFR) kinase inhibitors are effective treatments for lung cancers with EGFR activating mutations, but the magnitude of tumor regression varies and drug resistance is unavoidable. Multiple mechanisms of resistance to EGFR-TKIs have been identified, including the occurrence of secondary mutations in the EGFR gene, MET amplification, acquired BRAF rearrangements and activation of bypass pathways. Central to these mechanisms of resistance is the re-activation of AKT and ERK signaling, which enables escape of tumor cells from EGFR inhibitor treatment. However, the mechanisms of reactivation of PI3K-AKT and ERK/MAPK pathway are unclear.
Pleckstrin homology (PH) domain leucine-rich repeat protein phosphatase (PHLPP) acts as tumor suppressors in various types of human cancer by suppressing cell survival pathways and promoting apoptosis through inhibiting AKT and ERK pathway activation. Here, we hypothesize that PHLPP is a key regulator of EGFR-TKI resistance in lung cancer and a potential treatment target for overcoming resistance to EGFR-TKI treatment.
Method
A transcriptomic of PHLPP1 in non-small lung cancer cell according to gefitinib sensitivity obtained from Gene Expression Omnibus (GEO) database under accession number GSE4342 were analysis. The lentivirus-mediated delivery of shRNA was used to generated stable knockdown of PHLPP1 expression lung cancer cells, and retrovirus-mediated delivery was used to generated stable overexpression of PHLPP1 lung cancer cells. Western blotting, real-time PCR (RT-PCR) and immunofluorescence were used to determined PHLPP expression in vitro. PHLPP1 expression in clinical sample was determined by Immunohistochemical (IHC) staining. MTT assay was conducted to determine the cell proliferation. Xenografts bearing PHLPP overexpression and control were evaluated EGFR-TKI induced tumor regression.
Result
PHLPP1 gene expression was higher in gefitinib-sensitive NSCLC cell lines than gefitinib-resistant NSCLC cell lines from a GEO public database. In vitro, EGFR mutated NSCLC cell line HCC827 continuously exposing to gefitinib exhibited dramatically reduced expression of PHLPP1 and increased phosphorylation AKT and ERK. Knockdown of PHLPP1 decreased cell death induced by the EGFR-TKI in EGFR-mutant lung cancer cells, overexpression of PHLPP1 enhanced gefitinib-induced apoptosis in gefitinib-resistance EGFR-mutant lung cancer cells. In xenograft model, overexpression of PHLPP showed significantly more tumor regression after gefitinib treatment at 1-week time point compared to control group. In patients, PHLPP1 were highly expressed in tumors with EGFR common mutations pre- and post-development of resistance to EGFR TKIs. PHLPP1 expression were down regulated in the post-relapse tumor samples compared to that of pre-treatment, and patients with higher PHLPP1 expression in pre-treatment had significantly longer progression-free survival (PFS).
Conclusion
PHLPP loss may be a key molecule contributing to the resistance of EGFR-TKI by activating PI3K-AKT and ERK/MAPK signaling pathway. PHLPP may serve as a potential predictor of EGFR-TKI treatment response in these patients. Up-regulating PHLPP expression may prevent or /and delay the emergence of EGFR-TKI resistance. Further study is warranted to prove PHLPP as an effective strategy.
-
+
P2.12 - Small Cell Lung Cancer/NET (ID 180)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Small Cell Lung Cancer/NET
- Presentations: 1
- Now Available
- Moderators:
- Coordinates: 9/09/2019, 10:15 - 18:15, Exhibit Hall
-
+
P2.12-08 - Surprisingly Promising Tumor Control Rate of S1 Combination with Anlotinib with Refractory Relapsed SCLC Who Failed ≥ 2 Lines Chemotherapy (Now Available) (ID 885)
10:15 - 18:15 | Author(s): Suna Zhou
- Abstract
Background
Patients with refractory relapsed small cell lung cancer (SCLC) who failed more than 2 lines chemotherapy have limited options. Objective Response Rate (ORR) of Immunotherapy (CheckMate-032 and Keynote-158) was only 20%. Anlotinib is a novel TKI on multi-kinase (VEGFR, c-Kit, PDGFR, FGFR) angionesis inhibitor, had ORR of only 4.9% in ALTER1202 for the third-line and further-line treatment of SCLC. S-1, a combination of three pharmacological compounds, namely tegafur (prodrug of 5-fluorouracil), gimeracil, and oteracil potassium, is a new oral fluoropyrimidine derivative designed to enhance anticancer activity and reduce gastrointestinal toxicity, has been used for treatment of SCLC with ORR of 4% (MOLECULAR AND CLINICAL ONCOLOGY 1: 263-266, 2013). In patients without any option of systemic therapy, our clinic started combined use of these drugs. This study aimed to report the preliminary results of combined S1 and anlotinib therapy in these refractory released SCLC.
Method
This study retrospectively analyzed refractory relapsed in SCLC who failed to more than 2 lines’ chemotherapy. Eligible patient must have received Anlotinib (12 mg PO QD from day 1 to 14, every 3 weeks) and S1(60mg PO Bid from day 1 to 14, every 3 weeks) combined therapy. The primary endpoints were ORR and disease control rate (DCR). The secondary endpoints were PFS, OS, and safety and tolerability.
Result
A total of 12 patients were recruited from Nov 2018 in this study. There were 2 females and 10 males. The median age was 64 years (37-75 years). 6 patients had failed 2 lines of refractory diseases and 6 cases failed 3 lines’s chemotherapy. Until 31 March 31, 2019, 2, 3, 3, 2, and 2 cases had accomplished 6, 5, 4, 3, and 2 cycles, respectively. The ORR and DCR were 50% and 100%, respectively (Figure 1). The median PFS and OS were not reached at the time of data analysis. The most common Treatment-related adverse events (TRAEs) were hypertension, anorexia, fatigue, blurred vision, and hand-foot syndrome. Grade ≥3 TRAEs occurred in 5 (41.7%) of patients. Anlotinib was reduced to 8mg in 2 patients and 10mg in 3 patients due to grade 3 TRAEs.

Conclusion
The study demonstrates that S1 combination with anlotinib seemed to be an effective treatment option for patients with surprisingly promising response rate in refractory relapsed SCLC. Prospective clinical trial (SALTER Trial, ClinicalTrials.gov ID: NCT03823118) is ongoing to confirm the promising results.


