Virtual Library
Start Your Search
Pierre P Massion
Author of
-
+
EP1.11 - Screening and Early Detection (ID 201)
- Event: WCLC 2019
- Type: E-Poster Viewing in the Exhibit Hall
- Track: Screening and Early Detection
- Presentations: 1
- Now Available
- Moderators:
- Coordinates: 9/08/2019, 08:00 - 18:00, Exhibit Hall
-
+
EP1.11-24 - The TREAT Model 2.0: Predicting Lung Cancer in Patients Seeking Care in High-Risk Clinics (Now Available) (ID 2713)
08:00 - 18:00 | Author(s): Pierre P Massion
- Abstract
Background
Appropriate risk-stratification of indeterminate pulmonary nodules (IPNs) is necessary to estimate the best diagnostic strategy. Validated models for patients with high-risk IPNs are poorly calibrated. We sought to expand our previous Thoracic Research Evaluation And Treatment (TREAT) model into a more generalized, robust model for lung cancer prediction, the TREAT 2.0.
Method
A total of 1402 patients with known or suspected lung cancer were used to recalibrate the TREAT 1.0 model. Clinical data and patient demographics were retrospectively collected from six clinics located in four U.S. states. Six datasets were divided into 3 clinical groups: patients who presented to a pulmonary nodule clinic (n=375), patients who presented to an outpatient thoracic surgery clinic (n=553) and patients who presented for surgical resection (n=474). A logistic regression model using multiple imputation was developed and validated. Model variables included age, body mass index, gender, smoking pack-years, size of nodule, spiculation, growth over time, location in upper lobe, prior cancer history, pre-operative FEV1, pre-operative symptoms, FDG-PET positivity, and clinical group. The discrimination and calibration of the TREAT 2.0 model was estimated and compared to two other common models for lung nodules, the Mayo Clinic and Herder models.
Result
Lung cancer prevalence was as follows: pulmonary nodule clinic 42%, thoracic surgery clinic 73%, and surgical resection cohort 90%. The strongest predictors of cancer were clinical group, age, nodule growth, PET positivity, and smoking pack-years. The median TREAT 2.0 area under the receiver operating curve (AUC) for the imputed dataset was 0.86 (95% confidence interval (CI), 0.86-0.87) and the Brier score was 0.13. The TREAT 2.0 model had better accuracy (p < 0.001) (Figure 1) and calibration than the Mayo Clinic (AUC =0.74 95% CI: 0.74-0.75; Brier score=0.21) or Herder models (AUC=0.75; 95%CI: 0.74-0.75 and Brier score=0.19).
Conclusion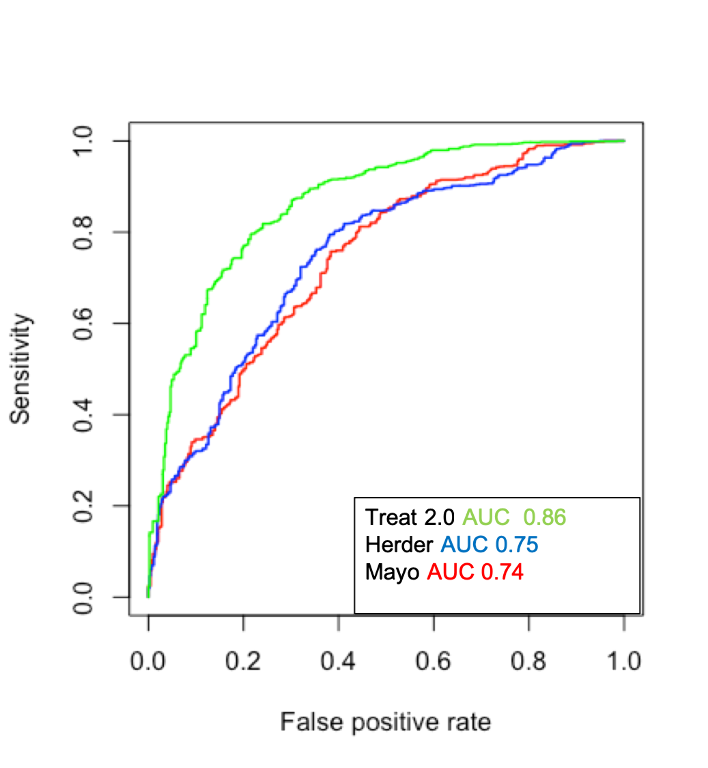
The TREAT 2.0 model is more accurate and better calibrated than the Mayo Clinic or Herder models in patients presenting with nodules at high risk for lung cancer. Nodule calculators such as the TREAT 2.0 that account for variation in lung cancer prevalence with a variable for clinical group may improve generalizability and increase use in clinical practice.
-
+
OA06 - Refining Lung Cancer Screening (ID 131)
- Event: WCLC 2019
- Type: Oral Session
- Track: Screening and Early Detection
- Presentations: 1
- Now Available
- Moderators:Tomasz Grodzki, Lluis Esteban Tejero
- Coordinates: 9/09/2019, 11:00 - 12:30, Hilton Head (1978)
-
+
OA06.06 - Independent Validation of a Novel High-Resolution Computed Tomography-Based Radiomic Classifier for Indeterminate Lung Nodules (Now Available) (ID 2862)
11:00 - 12:30 | Author(s): Pierre P Massion
- Abstract
- Presentation
Background
Optimization of the clinical management of incidentally- and screen-identified lung nodules is urgently needed to limit the number of unnecessary invasive diagnostic interventions, and therefore morbidity, mortality and healthcare costs. We recently developed and internally validated a novel radiomics-based approach for the classification of screen-detected indeterminate nodules, and present herein validation of this algorithm in an independent cohort.
Method
In a previous study, we developed a multivariate prediction model evaluating independent quantitative variables assessing various radiologic nodule features such as sphericity, flatness, elongation, spiculation, lobulation and curvature. Nodules between 7 and 30 mm of largest diameter were selected from the National Lung Screening Trial (n=726 indeterminate nodules, benign (n = 318) and malignant (n = 408)) were used to derive this model using least absolute shrinkage and selection operator (LASSO) method with bootstrapping method applied for the internal validation. Eight variables capturing vertical location, size, shape, density and surface characteristics were included with an optimism-correct area under the curve (AUC) of 0.94. For this study, an independent dataset of 203 incidentally-identified lung nodules obtained from the indeterminate pulmonary nodule registry at Vanderbilt University was identified. CT datasets were transferred to Mayo Clinic (Rochester, MN) for analysis. Nodules were segmented manually using the ANALYZE software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN), and radiomic analysis was performed using the 8-variable radiomic diagnostic algorithm derived from the NLST. The Brock model was also used to calculate probability of malignancy for all NLST and Vanderbilt nodules.
Result
Brock scores were calculated for 685 NLST nodules (excluded: interval cancers, n=12; missing values needed for Brock score, n=29). The AUC for the Brock score (AUC Brock) for NLST nodules was 0.83 which was inferior to the AUC for the radiomic model (AUC Radiomic =0.94, P<0.001). When the subset of intermediate pre-test probability of lung cancer was considered (Brock score > 10 but <= 60), the AUC Brock was 0.61 (95% CI: 0.54-0.68) whereas the AUC Radiomic was 0.88 (95% CI: 0.84-0.93). A total of 203 incidentally found pulmonary nodules with available clinical information and biopsy or surgery-proven histology identified in the Vanderbilt indeterminate pulmonary nodule registry, and all histology data and corresponding CT images were reviewed. CT images were transferred to Mayo Clinic for analysis. After exclusion of duplicate CT datasets, unanalyzable CT images and not identifiable nodules (n=27 cases), 176 nodules were segmented and analyzed, including 84 benign and 92 malignant nodules. The AUC was 0.89 (95% CI: 0.85-0.94). For comparison, the AUC Brock was 0.88 (95% CI: 0.83-0.94). When the subset of intermediate pre-test probability of lung cancer was considered (Brock score > 10 but <= 60), the AUC Brock was 0.76 (95% CI: 0.63-0.89) whereas the AUC Radiomic was 0.85 (95% CI: 0.74-0.95).
Conclusion
Our radiomic classifier demonstrates good performance characteristics on an independent retrospective validation dataset. If prospectively validated, integration into clinical decision making algorithm could significantly impact patient care.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.11 - Screening and Early Detection (ID 177)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Screening and Early Detection
- Presentations: 2
- Now Available
- Moderators:
- Coordinates: 9/08/2019, 09:45 - 18:00, Exhibit Hall
-
+
P1.11-02 - Acceleration of Lung Cancer Diagnosis: Utility Study for AI-Based Stratification of Pulmonary Nodules (ID 2752)
09:45 - 18:00 | Author(s): Pierre P Massion
- Abstract
Background
Lung cancer diagnostic pathway guidelines promote the use of risk stratification models. Artificial Intelligence (AI)-based risk models have been shown to achieve better diagnostic accuracy than clinical models like Mayo Clinic (Mayo) for particular clinical populations. The aim of this study is to examine whether this could translate into faster diagnosis for high-risk cancer patients.
Method
116 patients (116 nodules) have been collected from a retrospective consecutive cohort acquired at Vanderbilt University Hospital. Time to diagnosis (TTD) was defined as the number of days between the CT scan and diagnosis date. Mean TTD was calculated on the cohort on which TTD could be defined, and on a reduced group comprising of TTD >31 days only.
Risk scores for each nodule were found using the Mayo model and an AI-based Lung Cancer Prediction model (LCP) based on CT images alone. A 65% risk of cancer was taken to be the threshold at which surgical intervention is indicated (according to ACCP guidelines).
Result
Seven patients were dropped due to negative TTD, and six for having no definitive diagnosis date. The eventual cohort contained 61 cancer patients and 42 controls. Mean TTD is 140 days (Interquartile Range – IQR 1-77 days). 25 patients have TTD=0, 60 are within 31 days (28 cancers, 32 controls) and 43 (33 cancers, 10 controls) are above 32 days.
On the full cohort: Mayo risk score is >=65% for 15 cancers and 4 controls (sensitivity, 24.6%, specificity 90.5%), with a mean cancer TTD of 75 days. The LCP scores >=65% in 43 cancers and 10 controls (sensitivity, 70.5%, specificity 76.2%), mean cancer TTD 81 days.
On the reduced group: Mayo >=65% for 7 cancers and 2 controls (sensitivity, 21.2%, specificity 80.0%) with mean cancer TTD 150 days. The LCP scores >=65% in 21 cancers and 4 controls (sensitivity, 63.6%, specificity 60.0%), with mean cancer TTD 156 days.
Conclusion
The LCP risk model could potentially accelerate the diagnosis in 40% more cancer patients who were not worked up fully in the month following a scan (the jump in sensitivity going from Mayo to LCP risk >=65% is 42.4%). For these patients, time to a cancer diagnosis and treatment could be shortened by up to 156 days compared to recommendations if applying the Mayo risk model.
-
+
P1.11-31 - Understanding Referral Patterns in a Lung Cancer Screening Program (Now Available) (ID 726)
09:45 - 18:00 | Author(s): Pierre P Massion
- Abstract
Background
Lung cancer screening (LCS) provides an important way to reduce the morbidity and mortality of lung cancer, however, the uptake of LCS has remained strikingly low, which has been attributed to both patient and provider level factors. We examined referring clinician and patient characteristics to direct interventions to promote LCS.
Method
We examined the sociodemographic and geophysical characteristics of patients and their initial referring providers at a single institution LCS program from 1/1/2015-7/15/2018. Using data from the clinical record, we geocoded patients, generated maps, and estimated area deprivation indices, a proportional estimate of community socioeconomic status incorporating education, employment and poverty, at the census track level. We performed descriptive analysis of patient level factors such as race, insurance status, and their distribution across referring providers.
Result
1085 unique individuals—909 (84.0%) whites, 143 (13.2%) blacks, and 30 others (3.7%) were referred for LCS by 268 unique providers with 36 cancers detected. Approximately half (137) of providers referred one only individual and 27(10.1%) referred 52% of the patients screened. Primary care providers consisted of 76.4% of referrers, followed by pulmonologists (7.6%) and oncologists (5.3%). 211 referrers (78.7%) were affiliated with our institution. Referral of non-white patients was uniformly distributed among 170 referring providers. Referrals were geographically clustered in counties neighboring the hospital (Figure 1). Average area deprivation was 0.347 with a broad range (0.0770-0.838), with no difference between whites and blacks (0.345 vs 0.354, p=0.482), despite higher PLCO model estimated risk in blacks(7.1 vs 4.9, p<0.001).
Conclusion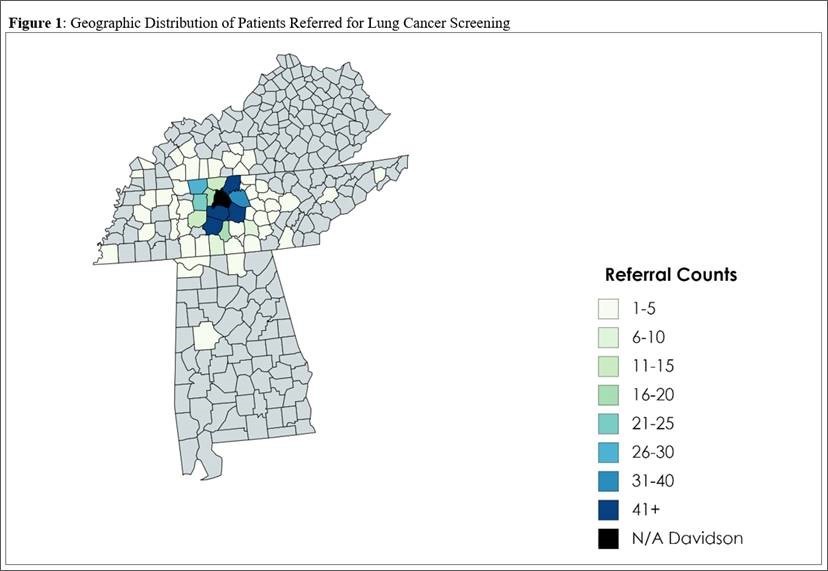
A small number of physicians referred the majority of patients for LCS, however the distribution of minorities was nearly uniform across referrers. We demonstrate broad knowledge of LCS by the large number of referrers, in spite of low referral volume by most providers. These results are guiding ongoing initiatives to improve the overall uptake of LCS by targeted provider communication and community education strategies.
-
+
P2.05 - Interventional Diagnostic/Pulmonology (ID 168)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Interventional Diagnostics/Pulmonology
- Presentations: 1
- Moderators:
- Coordinates: 9/09/2019, 10:15 - 18:15, Exhibit Hall
-
+
P2.05-01 - Broad Genomic Profiling of Bronchoalveolar Lavage Fluid in Lung Cancer (ID 615)
10:15 - 18:15 | Author(s): Pierre P Massion
- Abstract
Background
We hypothesized that tumor-derived mutations from non-small cell lung cancer (NSCLC) are readily detectable in bronchoalveolar lavage (BAL). To explore our hypothesis, we employed the CAncer Personalized Profiling by deep Sequencing (CAPP-Seq) method to identify somatic mutations in BAL compared to blood.
Method
We profiled 200 matching lavage, plasma, and PBMC samples from a total of 38 NSCLC patients and 21 controls. We first applied a tumor-informed calling approach to most sensitively detect mutations in BAL and plasma. We then applied a tumor-naïve mutation calling strategy to explore the effect of field cancerization in at risk patients with lung nodules or who smoked. Last, we developed a BAL mutation classifier to differentiate patients with cancer from those without and compared the performance of this classifier to BAL cytology.
Result
Tumors were primarily lung adenocarcinomas (84%) and mostly early stage disease (I-II 71%; III-IV 29%). We called a median of 4 mutations per tumor. TP53 and KRAS were the most frequently detected variants in tumor (47% and 35% respectively) and lavage cell free (cf) DNA (38% and 26% respectively). Using a tumor-informed approach, we detected significantly more variants in lavage cfDNA than in plasma from cancer patients (p<0.001) and variants were more frequently called in lavage cfDNA than in plasma from cancer patients (77% vs. 41%, p=0.004). As expected, tumor-naïve calling resulted in fewer variants detected in both sample types when compared to tumor-informed calling but we identified more tumor mutations (p<0.001) and more putative cancer driver mutations (p< 0.003) in lavage cfDNA than in plasma. Mutations of cancer driver genes at the patient level and average %VAF at the gene level were significantly lower in lavage cfDNA controls compared to cancer patients (p=0.017 and p=0.016 respectively). Since we also detected mutations in controls, presumably secondary to field cancerization and somatic mosaicism, we developed a risk score of mutation features to classify whether a BAL specimen was likely to come from a cancer patient or control. At a risk score level that identified all non-cancers as benign (A), this BAL classifier of 11 mutation features identified more cancers than cytology for all stages of lung cancer (65% vs 12%, p=0.001) and in stage I/II disease (50% vs. 20%, p=0.25; B).
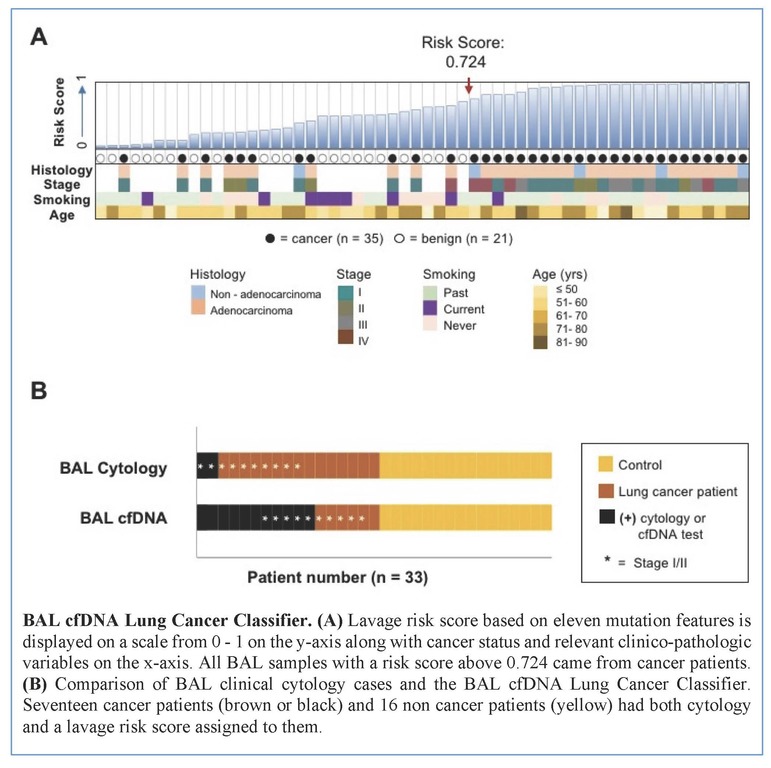
Conclusion
We show here that BAL genomic profiling may augment plasma profiling and BAL cytology for diagnosing and profiling NSCLC. Validation studies will be required to confirm our findings.
-
+
P2.11 - Screening and Early Detection (ID 178)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Screening and Early Detection
- Presentations: 2
- Moderators:
- Coordinates: 9/09/2019, 10:15 - 18:15, Exhibit Hall
-
+
P2.11-01 - Novel Flexible Longitudinal Machine Learning Coupled with Patient Demographics Improves Lung Cancer Risk Prediction Using Whole Screening CTs (ID 2856)
10:15 - 18:15 | Author(s): Pierre P Massion
- Abstract
Background
The inherent variability of screening scans in clinical practice limits existing machine and deep learning techniques, who have difficulty accessing all available information. As a result, cohorts are less generalizable or require integrating human intervention to initiate data definition. We created a deep learning prediction model for lung cancer screening that could define temporally static and time varying imaging features and combine them with common epidemiological factors collected at the time of shared decision making. We propose a new longitudinal lung cancer detection method, called longitudinal imaging and clinical data co-learning (LICDC), which integrates the temporally flexible deep imaging features and clinical features from the PLCO model.
Method
722 individuals with cancer and 1072 random participants without cancer with more than one scan were selected from the NLST. We initiated the deep learning model using Kaggle contest algorithm discovered features for cancer using each CT scan (n=4781) in its entirety as an independent observation (temporally varying features are not created). We then applied our flexible long and short-term temporal memory methodology to extract additional longitudinal features and generate a neural network model. This training for the LICDC model used NLST participants (n=829) and scans (n=3588) and 826 participants (367 had multiple scans) with 1193 scans from our local screening program. Ten-fold cross-validation was performed on a participant basis. Cancer risk probabilities from imaging-only deep learning model (DLSTM) were combined with PLCO model predictions and regressed together (LICDC-Logistic Regression). Additionally, individual variables from the PLCO model and the cancer probability from DLSTM were fitted with support vector machine (SVM) method using linear kernels (LICDC-SVM) and their area under the receiver operating curve was estimated (AUC). All AUC curves were calculated from the same 1655 patients (829 from NLST and 826 from our local screening program).
Result
PLCO’s predicted risk and basic imaging features (Kaggle winner) had similar accuracy with AUC of 0.815 and 0.781, respectively. Combining risk estimates from the full longitudinal deep learning and PLCO model in a simple logistic regression increased AUC to 0.861. Re-estimation of PLCO and imaging features LICDC-SVM further increased AUC to 0.918.
Conclusion
 Risk prediction for lung cancer in patients who are eligible for screening is improved by combining longitudinal machine learning CT imaging data with demographic information. These methods allow for risk prediction based on a complete CT imaging data set with varying time between scans and across participants without relying on a segmented nodule.
Risk prediction for lung cancer in patients who are eligible for screening is improved by combining longitudinal machine learning CT imaging data with demographic information. These methods allow for risk prediction based on a complete CT imaging data set with varying time between scans and across participants without relying on a segmented nodule. -
+
P2.11-33 - Organizational Readiness for Implementation of Lung Cancer Screening in a Veterans Affairs Healthcare System (ID 709)
10:15 - 18:15 | Author(s): Pierre P Massion
- Abstract
Background
Implementation of high quality lung cancer screening is complex and requires close coordination between radiology and primary care teams. Organizational readiness for change (ORC) is an important factor in successful implementation of complex healthcare programs such as lung cancer screening. Using the Consolidated Framework for Implementation Research (CFIR), we tested the hypothesis that ORC would differ between radiology and primary care prior to deployment of a centralized lung cancer screening program.
Method
We conducted a cross-sectional observational study. We invited all radiology and primary care providers (hospital and community-based) and affiliated staff at a single large VA Healthcare System in the US by email to participate in a web-based survey. We measured demographic information and adapted 9 validated items on ORC (domains of change commitment and change efficacy) and 10 items on change valence (value of a planned organizational change) using a 7-point Likert-type scale. Respondents’ ORC and change valence scores were calculated by averaging individual item responses for each scale. The primary outcome, ORC, was evaluated as a continuous variable with higher scores representing more readiness. We compared mean ORC scores between radiology and primary care using independent 2-sample t-tests.
Result
The overall response rate was 54% (76/128 [59.4%] radiology, 206/398 [51.8%] primary care). After 12 respondents were excluded for incomplete data (5 from radiology and 7 from primary care), the analytical sample was 270 respondents. Respondents were on average 47 years old [SD 11.24], 72% female, and 17% self-identified as having a leadership role. Individuals affiliated with radiology reported higher ORC than those affiliated with primary care (5.50 [SD 1.42] versus 5.07 [SD 1.22], p=0.03). Individuals self-identifying as having leadership roles in implementation of lung cancer screening reported higher ORC than those without leadership roles (5.56 [SD 1.38] vs 5.11 [SD 1.28], p=0.05). Those with leadership roles reported higher change valence than those without (5.91 [SD 1.20] vs. 5.36 [SD 1.88], p=0.006). We found no difference in reported change valence between radiology and primary care.
Conclusion
Radiology providers and staff have higher perceived ORC to implement a centralized lung cancer screening program compared to primary care. Providers and staff with implementation leadership roles reported higher ORC than those without leadership roles. Understanding these differences in readiness will inform future work as we focus on strategies to engage primary care providers and staff during implementation of lung cancer screening. We will deploy these strategies at Veterans Health Administration facilities across the US with the support of the VA-Partnership to increase Access to Lung cancer Screening (VA-PALS) and the VA Office of Rural Health.


