Virtual Library
Start Your Search
Noriko Motoi
Moderator of
-
+
MA15 - Usage of Computer and Molecular Analysis in Treatment Selection and Disease Prognostication (ID 141)
- Event: WCLC 2019
- Type: Mini Oral Session
- Track: Pathology
- Presentations: 12
- Now Available
- Moderators:John Le Quesne, Noriko Motoi
- Coordinates: 9/09/2019, 15:45 - 17:15, Tokyo (1982)
-
+
MA15.01 - Cellular Prion Protein Transcriptionally Regulated by NFIL3 Enhances Lung Cancer Cell Lamellipodium Formation and Migration (Now Available) (ID 151)
15:45 - 17:15 | Presenting Author(s): Shin-Chih vincent Lin | Author(s): Chia-Hung Lin, Nien-Chu Shih, Hsin-Ling Liu, Wen-Chao Wang, Kun-Yang Lin, Yi-Cheng Lin, Yi-Chen Yeh, Hiroshi Minato, Takeshi Fujii, Yu-Chung Wu, Mei-Yu Chen, Teh-Ying Chou
- Abstract
- Presentation
Background
Tumor invasion and metastasis are the major causes of treatment failure and mortality in lung cancer patients. However, the precise molecular targets responsible for tumor invasion remain unclear.
Method
In this study, we identified a group of genes with differential expression in in situ and invasive lung adenocarcinoma tissues by cDNA microarray analysis; among these genes we further characterized the association of the upregulation of PRNP, the gene encoding cellular Prion protein (PrPc), with lung adenocarcinoma invasiveness through immunohistochemistry and in situ hybridization analysis on clinical tissues. The roles of PrPc in lung cancer cell lines were also verified by using immunofluorescence staining, in vitro transwell assay and in vivo metastasis mouse model. In addition, the impact of PrPc on the activation of the JNK signaling pathway was investigated by Western blot analysis. Finally, luciferase reporter assay and chromatin immunoprecipitation assay were used to identify the transcriptional activators of PRNP.
Result
Immunohistochemistry on clinical specimens showed association of PrPc expression with invasive but not in situ lung adenocarcinoma. Consistently, the expression of PrPc was higher in the highly invasive than in the lowly invasive lung adenocarcinoma cell lines. Knockdown of PrPc expression in cultured lung adenocarcinoma cells decreased their lamellipodium formation, in vitro migration and invasion, and in vivo experimental lung metastasis. Phosphorylation of JNKs was found to correlate with PrPc expression and the inhibition of JNKs suppressed the PrPc-induced up-regulation of lamellipodium formation, cell migration, and invasion. Moreover, we identified the nuclear factor, interleukin 3 regulated (NFIL3) protein as a transcriptional activator of the PRNP promoter. Accordingly, NFIL3 promoted lung cancer cell migration and invasion in a PrPc-dependent manner. High NFIL3 expression in clinical specimens of lung adenocarcinoma was also associated with tumor invasiveness and poor survival of patients.
Conclusion
Our observations suggest that PRNP expression is associated with the invasiveness of lung adenocarcinoma, and cell line model demonstrated that PrPc serves as a critical factor for lung cancer cell lamellipodia formation, migration and invasion via JNK signaling. A novel transcription factor, NFIL3, was identified to upregulate PRNP expression in lung cancer cells; further characterizations showed that NFIL3 promotes lung cancer cell migration through PrPc-dependent manner. Moreover, high NFIL3 expression was found to be associated with lung cancer invasiveness in clinical tissues. Overall, NFIL3/PrPc axis plays a critical role in lung cancer invasiveness and metastasis, and may be the potential therapeutic targets in the future.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.02 - Deep Learning Approach for Automated Tumor Cells Detection and Estimation of PD-L1 22C3 Assay Expression in Lung Adenocarcinoma (Now Available) (ID 577)
15:45 - 17:15 | Presenting Author(s): Jianghua Wu | Author(s): Dongmei Lin, Wei Sun, Changling Liu, Xiaoqing Liu, Yajun Zhang, Junjie Zhang, Haiyue Wang, Xinying Liu, Xin Yang, Shaoping Ling
- Abstract
- Presentation
Background
It is vital and challenging to assess an accurate PD-L1 expression status on tumor cells for immunotherapy in lung cancer. The purpose of this study was to set up an automated system to detect the tumor cells and estimate the tumor proportion score (TPS) of PD-L1 immunohistochemistry (IHC) expression for lung adenocarcinoma based on deep learning, and provide a potential Artificial Intelligences (AI) assistive diagnostic tool in the quantification of PD-L1 interpretation.
Method
Fifty PD-L1 22C3 IHC slides of lung adenocarcinoma samples on digitized whole-slide images (WSI) database was employed. We first designed a model with a fully convolutional neural network (FCNN) based on U-ResNet architecture to obtain the cancer segmentation. Representative regions were selected from each slide, and 100 regions were collected for manual annotations as a training set for cancer detection. Another 50 regions were used to validate the performance of automated cancer detection and TPS estimation as a test set. After the quality control, a whole model of automated cancer cell segmentation and membrane positive estimation was set up on standard PD-L1 22C3 IHC staining. TPS could be automatically predicted by AI tool and then compared with the interpretations of pathologists.
Result
The results of automated lung adenocarcinoma cells segmentation on the test set of 22C3 IHC staining showed a moderate sensitivity (71.46%) with a high specificity (95.94%) which was much more crucial for TPS counting. In rest 43 out of 50 regions after a quality control, TPS estimated by the automated PD-L1 analysis based on cancer segmentation showed a significant correlation with the average scores (r=0.9609, p<0.001) and the median scores (r=0.9523, p<0.001) of pathologists' interpretations.

Conclusion
We provide an automated tumor cells detection and TPS estimation model for lung adenocarcinoma and demonstrate the potential of using machine learning methods to access PD-L1 IHC status conveniently. A further validation of AI tool for automated scoring PD-L1 in diagnostic routine is highly recommended in the future.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.03 - Exploring Digital Pathology-Based Morphological Biomarkers for a Better Patients’ Selection to the Immune Checkpoint Inhibitor of Lung Cancer (Now Available) (ID 1777)
15:45 - 17:15 | Presenting Author(s): Noriko Motoi | Author(s): Hiroshi Yoshida, Tomoharu Kiyuna, Hidehito Horinouchi, Takashi Kohno, Shun-ichi Watanabe, Yuichiro Ohe, Atsushi Ochiai
- Abstract
- Presentation
Background
For eligible patients’ selection for immune checkpoint inhibitor therapy (ICI), it is important to establish more accurate predicting biomarkers, in addition to PD-L1 IHC and MSI-high. We hypothesized that morphological characteristics should reflect genetic alteration, thus could predict ICI responsiveness. In this study, we examined the predictive potential of morphological characteristics using digital whole-slide images as a new biomarker for ICI-treatment on non-small cell lung cancer (NSCLC) and their relationship to PD-L1 IHC and genetic alterations.
Method
71 NSCLC who received ICI therapy were recruited. Digital images of H&E and PD-L1 (22C3) IHC stained slides of pre-treatment biopsied or resected materials were examined by previously reported image analysis techniques using e-Pathologist ® (NEC, Japan). Morphological characteristics of cancer cells (three and six parameters of nuclear shape and chromatin texture) were extracted as MC-scores. Of 11 cases (pilot cohort), PD-L1 IHC (22C3) and tumor mutation burden (TMB) by the NGS-based target sequence (NCC oncopanel ®) were examined. Correlation between MC-score, PD-L1 IHC, TMB status, and clinical outcome was calculated. A p-value of less than 0.05 was defined as statistically significant.
Decision tree analysis for evaluating predicting ICI-responsiveness was built using statistically significant MC-scores. We also tested the predictive value of a deep learning analysis (AI model) with 5-fold cross-validation. AUC (area under the curve) of ROC analysis was calculated.
Result
Of the responders, the MC-score of cancer cell were statistically different from those of the non-responders; nuclear texture contour complexity (11.8 vs. 8.25, median value of responder vs. non-responders; p<0.01), homogeneity (0.396 vs. 0.421; p<0.01), angular second moment (ASM) (0.0203 vs. 0.0214; p=0.049) and nuclear circularity (0.878 vs. 0.885, p=0.026). Circularity (p=0.011) and texture homogeneity (p=0.048) correlated with TMB. ASM texture correlated with PD-L1 expression (p=0.018). The decision tree model for predictive and screening purposes resulted in 0.83 and 0.62 accuracies, respectively. AUC of AI-model for ICI responsiveness resulted in fair (0.74 on average, range 0.55-0.81).
Conclusion
Our results indicate the substantial value of the morphological feature as a biomarker for ICI therapy. Morphological characteristics are eligible from archived FFPE samples, showed good correlation to the underlying genetic alteration. Digital pathology can serve useful predictive morphological biomarkers for precision medicine of lung cancer patients, and promising the power of AI-assisted pathology.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.04 - Discussant - MA15.01, MA15.02, MA15.03 (Now Available) (ID 3780)
15:45 - 17:15 | Presenting Author(s): Anja C. Roden
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.05 - Computerized Measurements of Cellular Diversity on H&E Tissue Are Prognostic of OS and Associated with Mutational Status in NSCLC (Now Available) (ID 1975)
15:45 - 17:15 | Presenting Author(s): Vamsidhar Velcheti | Author(s): Cheng Lu, Kaustav Bera, Xiangxue Wang, Pingfu Fu, Michael Yang, David Rimm, Kurt A Schalper, Anant Madabhushi
- Abstract
- Presentation
Background
Tumor heterogeneity is known to be implicated in chemotherapeutic resistance and poor prognosis for non-small cell lung cancer (NSCLC). In this study we sought to evaluate the role of computer extracted features reflecting the intrinsic cellular morphological diversity (ICMD) of tumors from digitized H&E stained images of early-stage NSCLC patients. Additionally, we sought to evaluate the association of these ICMD features in adenocarcinomas with the ALK and EGFR mutational status.
Method
Two cohorts, D1 and D2, of digitized H&E stained tissue microarray images (TMA) of NSCLC, n=395 and n=91, respectively, were used for modeling the ICMD predictor. A pretrained deep learning model was used for segmentation of nuclei, and clusters of proximally located nuclei were identified. The ICMD features were then extracted as the variations in shape, size, and texture measurements of nuclei within the clusters. A Cox proportional hazard model using the ICMD features was then trained for lung adenocarcinomas (LUAD, n=270), and squamous cell carcinomas (LUSC, n=216), separately, and was validated on independent cohort from (D3) The Cancer Genome Atlas (TCGA) (n=473) to predict Overall Survival (OS). Univariate and multivariate analyses were performed on (D3).
Result
In (D3), high risk patients predicted by the ICMD features had significantly poorer survival (HR (95% CI) = 1.48 (1.06-2.06), p=0.021 for LUSC, HR (95% CI) = 1.59 (1.11-2.29), p=0.006 for LUAD) in univariate analysis. In multivariate analysis, controlling for major clinical variables, ICMD was independently associated with 5-year OS (p<0.016). (See Table 1) We also found that ICMD features were associated with driver mutations ALK (p=0.0204) and EGFR (p=0.0017) in LUAD.
Table 1| Multivariate analysis for overall survival on the validation set D3.
ConclusionMultivariate Cox Proportional Hazard Model Analysis Controlling for Other Variables
TCGA-LUSC
TCGA-LUAD
Variable
HR (95% CI)
p value
HR (95% CI)
p value
Age (>65 vs <=65)
1.14(0.81-1.61)
0.451
0.89(0.63-1.28)
0.540
Smoking status
1.36(0.83-2.23)
0.221
1.14(0.64-2.01)
0.661
Overall Stage (Stage II vs I)
1.13(0.66-1.94)
0.651
1.86(1.04-3.32)
0.037
T-Stage (T2,3 vs T1)
1.26(0.85-1.87)
0.244
1.25(0.85-1.85)
0.263
N-Stage (N1 vs N0)
1.36(0.77-2.41)
0.292
3.11(1.55-6.23)
0.001
Developed Model
High risk vs. Low risk
1.52(1.08-2.13)
0.016
1.55(1.09-2.22)
0.015
CI = 95% confidence interval; HR = Mantel-Haenszel Hazard ratio. Values in bold are statistically significant, p<=0.05.
Computer extracted image features of cellular diversity were able to predict OS in NSCLC and were also associated with the ALK and EGFR mutational status. Future work will entail evaluating ICMD features in predicting added benefit of adjuvant therapy in early stage NSCLCs as well as correlating with gene expression data.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.06 - Stage I Lung Adenocarcinoma Gene Expression Associated with Aggressive Histologic Features for Guiding Precision Surgery and Therapy (Now Available) (ID 1124)
15:45 - 17:15 | Presenting Author(s): Jiarui Zhang | Author(s): Eric Burks, Travis Sullivan, Jacob Sands, Shawn Regis, Brady McKee, Andrea McKee, Sherry Zhang, Hanqiao Liu, Gang Liu, Steven Dubinett, Avrum Spira, Jennifer Beane, Kimberly Christ, Marc Lenburg
- Abstract
- Presentation
Background
Stage I lung adenocarcinomas (LUADs) show heterogeneity in histologic patterns which correlate with malignant behavior. Solid, micropapillary and cribriform patterns are associated with worse survival whereas lepidic (in situ) predominance has the best prognosis. In this study, we sought to characterize histologic pattern specific gene expression in resected clinical stage I LUADs. We also aimed to train and validate a genomic biomarker predictive of histologic aggressive patterns with the ultimate goal of being able to impact surgical and therapeutic decision making for post-biopsy management.
Method
A training cohort of 56 tumors from patients meeting NCCN high-risk screening criteria with stage I LUAD was included for pathologic annotation and whole exome RNA sequencing. Histologic pattern subtyping in 5% increments including all diagnostic slides was performed. A single representative FFPE block was chosen for RNA library-prep with Illumina TruSeq Access Kit and sequencing. Negative binomial models were used to identify gene expression differences associated with percent solid, cribriform, or micropapillary histology, and EnrichR was used for gene pathway enrichment analysis. Ss-GSEA was used to predict tumor infiltration of 20 immune cell types. A random-forest classifier for predicting aggressive histologic patterns was trained using 5-fold cross validation. A set of tumors from 16 independent patients with ≤2.0 cm clinical stage I LUAD was macro-dissected into 32 paired components (lepidic + non-lepidic regions) and subjected to RNAseq. Six tumors were defined as non-aggressive (lepidic + acinar/papillary) and ten tumors were defined as aggressive (lepidic + solid/micropapillary/cribriform). Four aggressive tumors were upstaged after surgical resection.
Result
In the training cohort, we identified 1322 genes associated with tumor histologic composition(FDR q <0.05 and fold-change > 2). Genes whose expression differs with solid histology% are enriched for involvement in DNA replication, cell cycle regulation and inflammation (FDR q<0.001). Genes whose expression is associated with micropapillary% are enriched for involvement in tRNA-aminoacylation and decrease of T-cell activity (FDR q<0.001). The functional enrichment of genes whose expression is associated with cribiform% was less informative. LUADs with micropapillary patterns exhibited gene expression consistent with decreased antigen presentation and low T-cell infiltration, and solid patterns exhibited gene expression consistent with increased infiltration of T-regulatory and Th2 cells (FDR q<0.05).
A gene expression classifier was trained to predict the presence of aggressive histologic patterns. We validated this classifier on a set of 16 tumor specimens from which we macro-dissected and analyzed tissue from the most aggressive histologic pattern (AUC = 1.00). We also found that this classifier could differentiate lepidic regions isolated from aggressive tumors from lepidic regions isolated from non-aggressive tumors (AUC = 0.74).
Conclusion
We identified solid-, micropapillary- and cribriform-specific gene expression and associated immune response among clinical stage I LUADs, and developed a classifier predictive of aggressive histologic features using either lepidic (in situ) or non-lepidic components. As such, this biomarker has the potential to predict histologic aggressiveness even from pre-surgical tumor biopsies where all histologic patterns may not be represented. Such a biomarker may be useful in guiding clinical decision making including extent of surgical resection.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.07 - Circulating miRNA: A Biomarker for Classification of Lung Cancer and Benign Lung Disease (Now Available) (ID 2218)
15:45 - 17:15 | Presenting Author(s): Alvida Qvick | Author(s): Anders Wirén, Maria Rönnqvist, Gisela Helenius
- Abstract
- Presentation
Background
Circulating biomarkers for cancer have great potential for diagnosis as well as follow up of treatment. MicroRNAs (miRNA) are involved in the expression of a majority of proteins with different cell types having different miRNA expression. The aim of this study was to create a circulating miRNA-based model to discriminate patients with lung cancer from patients with benign lung disease.
Method
Samples were collected from patients under investigation for lung cancer at Örebro University hospital. Patients were then divided into groups based on diagnosis, which resulted in NSCLC adenocarcinoma (n=24), NSCLC squamous cell carcinoma (n=13), SCLC (n=4) and a heterogeneous group consisting of different benign lung diseases (n=19). Healthy controls were collected separately (n=17).
Circulating miRNA was processed using the extraction-free library preparation miRNA Whole Transcriptome Assay with probes for 2083 human mature miRNAs and analyzed with massive parallel sequencing.
Differential expression between groups was estimated using edgeR. MiRNAs that had the highest impact on patient grouping were used in a sPLS discriminant analysis. The resulting classification model was validated using the leave-one-out method.
Result
The final model for comparison between patients with benign lung disease and patients with lung cancer contained 19 miRNAs. The model had an error rate of 15 % with errors distributed evenly between groups. A sub-analysis of patients with mutations in EGFR (n=5) and KRAS (n=6) was performed showing two distinct patterns in miRNA expression.
Conclusion
MiRNA shows promise as a circulating biomarker for lung cancer but may not be sufficient as an independent classifier. The predictive power may be improved by using several biomarkers in combination. The difference in expression between tumors with different mutations may be derived from alternate driving processes in these tumors.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.08 - Discussant - MA15.05, MA15.06, MA15.07 (Now Available) (ID 3781)
15:45 - 17:15 | Presenting Author(s): John Longshore
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.09 - PD-L1 Status in Relation with Non-Small Cell Lung Cancer Major Subtypes, Differentiation, Molecular Profiling and Smoking History (Now Available) (ID 2874)
15:45 - 17:15 | Presenting Author(s): Zhaolin Xu | Author(s): Alexi Surette, Mathieu Castonguay, Drew Bethune, Arik Drucker, Daniel French, Harry Henteleff, Mary Macneil, Wojciech Morzycki, Madelaine Plourde, Stephanie Snow
- Abstract
- Presentation
Background
Continued advances in lung cancer precision medicine have allowed targeted therapies based on an individual tumor’s genetic makeup. Recent advances in immune therapy based on immune checkpoint inhibitors have provided additional promising results. Currently, the majority of lung cancer mutational data available in the literature are from advanced stage non-small cell lung cancer. Mutational data from early stage lung cancer patients is limited. There is also limited data on PD-L1 tumor status is relation to mutational status along with other pathological and clinical characteristics. In this study, we evaluated these issues in 871 cases of surgically resected lung cancer.
Method
Multiplexed molecular profiling in 871 surgically resected lung cancer specimens was performed. A panel of genes including EGFR, KRAS, BRAF, PIK3CA, HER2 and ALK was tested. Tumor PD-L1 status was also evaluated by immunohistochemistry using pharmDx22C3. PD-L1 status was measured by tumor proportional score (TPS): <1%, 1-49% and ≥50% tumor cell positivity. Correlations between PD-L1 and gene mutation status, smoking history, histological grade, gender and age of paraffin embedded blocks were analyzed.
Result
This cohort includes adenocarcinoma (68%), squamous cell carcinoma (SCC) (22%) and other subtypes (10%). The average age is 67. Females account for 52%. A positive smoking history was present in 93%. Well differentiated tumors (G1) account for 11%, moderately differentiated (G2) 37% and poorly and undifferentiated (G3) 52%. EGFR mutations were identified in 7.4% and KRAS mutations in 31.7%. TPS <1% accounted for 48.8%, 1-49% for 34.6% and ≥50% for 16.5%.
There was no statistically significant difference in PD-L1 TPS between histological subtypes or gender. Significantly more G1 tumors had a TPS <1% (76.7%) compared to G2 (57.4%, p=0.0013) and G3 tumors (41.8%, p<0.0001). Fewer G1 tumors had a TPS 1-49% (20.9%) than G2 (34.1%, p=0.015) and G3 (35.2%, p=0.01) tumors. G3 tumors were more likely to have a TPS ≥50% (24.6%) than G1 (2.3%, p<0.0001) and G2 (7.63%, p<0.0001) tumors. Never smokers were more likely to have a TPS <1% (71.1% vs 50.6%, p=0.04) and less likely to have a TPS ≥50% (5.8% vs 16.5%, p=0.04). Tumors with EGFR mutation were more likely to have a TPS <1% than those without EGFR mutation (70.7% vs 47.3%, p=0.0003) and less likely to have a TPS 1-49% (20.0 vs 35.5%, p=0.011). Tumors with KRAS mutations were less likely to have a TPS <1% (36.6% vs 54.9%, p<0.0001) and more likely to have a TPS 1-49% (40.6% vs 31.5%, p=0.0086) and ≥50% (22.8% vs 13.6%, p=0.0007). PD-L1 IHC performed on blocks stored for 2 years or longer had a statistically significant higher rate of TPS <1% compared to blocks stored for less than 2 years.
Conclusion
This study provides information relating to the relationship between PD-L1 levels and tumor molecular profile, histological grade and patient demographics. Additionally, we raise the possibility of false negatives on IHC performed for PD-L1 on paraffin embedded blocks stored for 2 years or more.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.10 - Stromal Markers of Activated Tumor Associated Fibroblasts Predict Poor Survival and Are Associated with Necrosis in Non-Small Cell Lung Cancer (Now Available) (ID 2212)
15:45 - 17:15 | Presenting Author(s): Jordi Alcaraz | Author(s): Josep Lluís Carrasco, Millares Laura, Anabel Martínez-Romero, Julio Sánchez De Cos, Ramon Rami-Porta, Luis M Seijo, Jose Ramirez, Noemi Reguart, Esther Barreiro, Eduard Monsó
- Abstract
- Presentation
Background
Tumor associated fibroblasts (TAFs) are essential contributors of the progression of non-small cell lung cancer (NSCLC). Most lung TAFs exhibit an activated phenotype characterized by the expression of α-SMA and fibrillar collagens. However, the prognostic value of these activation markers in NSCLC remains unclear.
Method
We conducted a retrospective multicentric study of the prognostic value of the standard markers of activated fibroblasts. For this purpose, we conducted a quantitative image analysis of α-SMA immunostaining and picrosirius red staining of fibrillar collagens imaged by bright-field and polarized microscopy, respectively, using tissue microarrays with samples from 220 surgical patients, which elicited a percentage of positive staining area for each marker and patient.
Result
Kaplan-Meier curves showed that all TAF activation markers were significantly associated with poor survival, and their prognostic value was independent of TNM staging as revealed by multivariate analysis, which elicited an adjusted increased risk of death after 3 years of 129% and 94% for fibrillar collagens imaged with bright-field (p = 0.004) and polarized light (p = 0.003), respectively, and of 89% for α-SMA (p = 0.009). We also found a significant association between all TAF activation markers and tumor necrosis, which is often indicative of hypoxia, supporting a pathologic link between tumor desmoplasia and necrosis/hypoxia.
Conclusion
Our findings identify patients with large histologic coverage of fibrillar collagens and α-SMA+ TAFs to be at higher risk of recurrence and death, supporting that they could be considered for adjuvant therapy. Moreover it supports that antifibrotic drugs aiming to target tumor fibrosis may be an effective therapeutic approach to improve survival in NSCLC.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.11 - Establishing a Cell Sociology Platform for the Assessment of Targetable Interactions to Predict Lung Cancer Outcome (Now Available) (ID 2652)
15:45 - 17:15 | Presenting Author(s): Erin A Marshall | Author(s): Katey SS Enfield, Paul Gallagher, Martial Guillaud, Calum Macaulay, Wan Lam
- Abstract
- Presentation
Background
The tumor microenvironment (TME) is a complex mixture of tumor epithelium, stroma and immune cells. The immune component of the TME is highly prognostic for tumor progression and patient outcome. Immune functionality, however, is often dictated by direct cell-to-cell contacts and cannot be resolved by simple metrics of cell density (for example, number of cells per mm2 or flow cytometry). For example, direct contact between CD8+ T cells and target cells is necessary for CD8+ T cell activity, and direct contact between PD1+ and PD-L1+ cells is necessary for the efficacy of immune checkpoint inhibitors. Current immunohistochemistry (IHC) techniques identify immune cell numbers and densities, but lack assessment of spatial relationships (or “cell sociology”). Here, we develop a platform to examine these direct interactions within the TME, and assess their relationship with patient outcome in two independent non-small cell lung cancer (NSCLC) cohorts.
Method
Tissue sections of primary tumors from lung adenocarcinoma (LUAD) patients with known clinical outcome were stained using 2 multiplex IHC panels: CD3/CD8/CD79a (Panel 1) and PD1/PDL1/CD8 (Panel 2). Hyperspectral image analysis determined the phenotype of all cells. Using the same IHC panels, these observations were assessed in a secondary NSCLC dataset (n=674). Deconvolution of these images was used to identify cell types, and cellular ‘neighborhoods’ were assessed using a Voronoi approach. This cohort was also profiled by for gene expression to validate immune subset fractions. We further identified other tumor features, including the presence of tertiary lymphoid organs (TLOs; transient immune structures necessary for antibody production from B cells).
Result
High density of intra-tumoral CD8+ T cells was associated with non-recurrence of tumors. However, we find that a non-random cell sociology pattern of CD8+ T cells directly surrounded by tumor cells was more significantly associated with non-recurrence compared to density alone. Monte Carlo re‐sampling analysis determined that these cell sociology patterns were non-random.
Conclusion
Hyperspectral cell sociology expands our understanding of the complex interplay between tumor cells and immune infiltrate. This technology improves our understanding of the tumour microenvironment and allows us to directly quantify interactions that dictate immune responses to cancers. Consequently, the implementation of this platform could improve predictions of responses to immunotherapy and lead to a deeper understanding of anti-tumor immunity.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.12 - Discussant - MA15.09, MA15.10, MA15.11 (Now Available) (ID 3782)
15:45 - 17:15 | Presenting Author(s): David Hwang
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
Author of
-
+
EP1.18 - Treatment of Locoregional Disease - NSCLC (ID 208)
- Event: WCLC 2019
- Type: E-Poster Viewing in the Exhibit Hall
- Track: Treatment of Locoregional Disease - NSCLC
- Presentations: 1
- Now Available
- Moderators:
- Coordinates: 9/08/2019, 08:00 - 18:00, Exhibit Hall
-
+
EP1.18-03 - Outcome of Surgical Resection for Invasive Mucinous Adenocarcinoma: Experience at a Single Institution (Now Available) (ID 2105)
08:00 - 18:00 | Author(s): Noriko Motoi
- Abstract
Background
Invasive mucinous adenocarcinomas are new histological type which was determined by WHO classification 4thedition. The aim of this study was to investigate the outcome of surgical treatment for the patients with invasive mucinous adenocarcinoma who underwent surgery in our institution.
Method
Between 2010 and 2013, We identified 68 patients with invasive mucinous adenocarcinoma who underwent surgical resection. We measured the distance of tumor cell size and mucinous size, and their clinical and pathological data were retrospectively reviewed. Overall survival (OS) rates were compared using a log-rank test and survival curves were plotted using the Kaplan–Meier method.
Result
Participants comprised 31 men and 37 women, ranging in age from 46 to 83 years (median, 67 years). Median observation period in the survivors was 5.1 years. T classification of group oftumor cell size was T1/T2/T3,4=42/17/9 cases, and group of mucinous size was T1/T2/T3,4=38/17/13 cases. The 5-year overall survival rate for group oftumor cell sizewere T1/T2/T3,4=94.3/88.3/60.5%, and group of mucinous size were MS:T1/T2/T3,4=94.1/85.7/70.0%(5Y-OS tumor cell size / mucinous size : p=0.02/p=0.147, 5Y-RFS tumor cell size / mucinous size : p=0.04/p=0.237).
Conclusion
Our result indicated that to determine T classification, we should measure the distance of tumor cell size instead of mucinous size.
-
+
MA08 - Pawing the Way to Improve Outcomes in Stage III NSCLC (ID 127)
- Event: WCLC 2019
- Type: Mini Oral Session
- Track: Treatment of Locoregional Disease - NSCLC
- Presentations: 1
- Now Available
- Moderators:Simon Ekman, Helena A Yu
- Coordinates: 9/08/2019, 15:15 - 16:45, Tokyo (1982)
-
+
MA08.06 - Perioperative Outcomes of Lung Cancer Patients with Interstitial Pneumonia (Now Available) (ID 1372)
15:15 - 16:45 | Author(s): Noriko Motoi
- Abstract
- Presentation
Background
Interstitial lung disease is mostly found in elderly male smokers who also have relatively high risks of developing lung cancer. For these patients, modality to treat malignancy is limited to prevent acute exacerbation of interstitial pneumonia. We analyzed the perioperative outcomes of this group of patients with both interstitial pneumonitis and resectable lung cancer with curative intent.
Method
We retrieved the characteristics and medical courses of consecutive patients who had undergone pulmonary resections from medical records. In this analysis, usual interstitial pneumonia (UIP) was characterized by the presence of basal predominant, subpleural reticular abnormalities with traction bronchiectasis and honeycomb cysts detected in bilateral lung field on chest computed tomography preoperatively. Pathological findings on surgical specimen were used confirmation of diagnosis. The incidence and outcomes of acute exacerbation within 30 days from operation were analysed.
Result
From 2015 to 2017, there were 1,477 patients who underwent pulmonary resection for primary lung cancer at our institute. Among them there were 81 (5.5%) patients diagnosed as UIP by specific findings on chest computed tomography. Of 81 patients evaluated, 68 (84.0 %) were men, the median age was 73 years (range, 55-88). For Eastern Cooperative Oncology Group (ECOG) performance status, all 81 patients were categorized in status 0. Seventy-four patients (91.4%) underwent lobectomy, 1 (1.2%) bi-lobectomy, 2 (2.5%) segmentectomy and 4 (4.9%) wide wedge resections for primary lung cancer. The mean duration of surgery was 129 mins (range, 54-316), and mean value for blood loss was 36.5 ml (range, 0-396). A complete resection (R0) was achieved in 79 cases (97.5%). Postoperative complications were observed in 19 patients (23.5%) including prolonged air leakage (n=4, 4.9%), late onset of air leakage (n=3, 3.7%), surgical site infection, chylothorax and cerebral infarction. Nine patients (11.1%) manifested acute exacerbation of interstitial pneumonia within 30 days after surgery. There were 3 post-operative deaths (3.7%) within 30 days after surgery. Two deaths (2.5%) were due to acute exacerbation of interstitial pneumonia and 1 (1.2%) case of SAH on 1POD.
Conclusion
Pulmonary resection for patients with interstitial lung disease led to 9 (11.1%) cases of acute exacerbation within 30 days from surgery. Mortality related to acute exacerbation was found only 2 cases (2.5%) at our hospital, which was tolerable postoperative outcome for pulmonary resection for lung cancer with curative intent.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15 - Usage of Computer and Molecular Analysis in Treatment Selection and Disease Prognostication (ID 141)
- Event: WCLC 2019
- Type: Mini Oral Session
- Track: Pathology
- Presentations: 1
- Now Available
- Moderators:John Le Quesne, Noriko Motoi
- Coordinates: 9/09/2019, 15:45 - 17:15, Tokyo (1982)
-
+
MA15.03 - Exploring Digital Pathology-Based Morphological Biomarkers for a Better Patients’ Selection to the Immune Checkpoint Inhibitor of Lung Cancer (Now Available) (ID 1777)
15:45 - 17:15 | Presenting Author(s): Noriko Motoi
- Abstract
- Presentation
Background
For eligible patients’ selection for immune checkpoint inhibitor therapy (ICI), it is important to establish more accurate predicting biomarkers, in addition to PD-L1 IHC and MSI-high. We hypothesized that morphological characteristics should reflect genetic alteration, thus could predict ICI responsiveness. In this study, we examined the predictive potential of morphological characteristics using digital whole-slide images as a new biomarker for ICI-treatment on non-small cell lung cancer (NSCLC) and their relationship to PD-L1 IHC and genetic alterations.
Method
71 NSCLC who received ICI therapy were recruited. Digital images of H&E and PD-L1 (22C3) IHC stained slides of pre-treatment biopsied or resected materials were examined by previously reported image analysis techniques using e-Pathologist ® (NEC, Japan). Morphological characteristics of cancer cells (three and six parameters of nuclear shape and chromatin texture) were extracted as MC-scores. Of 11 cases (pilot cohort), PD-L1 IHC (22C3) and tumor mutation burden (TMB) by the NGS-based target sequence (NCC oncopanel ®) were examined. Correlation between MC-score, PD-L1 IHC, TMB status, and clinical outcome was calculated. A p-value of less than 0.05 was defined as statistically significant.
Decision tree analysis for evaluating predicting ICI-responsiveness was built using statistically significant MC-scores. We also tested the predictive value of a deep learning analysis (AI model) with 5-fold cross-validation. AUC (area under the curve) of ROC analysis was calculated.
Result
Of the responders, the MC-score of cancer cell were statistically different from those of the non-responders; nuclear texture contour complexity (11.8 vs. 8.25, median value of responder vs. non-responders; p<0.01), homogeneity (0.396 vs. 0.421; p<0.01), angular second moment (ASM) (0.0203 vs. 0.0214; p=0.049) and nuclear circularity (0.878 vs. 0.885, p=0.026). Circularity (p=0.011) and texture homogeneity (p=0.048) correlated with TMB. ASM texture correlated with PD-L1 expression (p=0.018). The decision tree model for predictive and screening purposes resulted in 0.83 and 0.62 accuracies, respectively. AUC of AI-model for ICI responsiveness resulted in fair (0.74 on average, range 0.55-0.81).
Conclusion
Our results indicate the substantial value of the morphological feature as a biomarker for ICI therapy. Morphological characteristics are eligible from archived FFPE samples, showed good correlation to the underlying genetic alteration. Digital pathology can serve useful predictive morphological biomarkers for precision medicine of lung cancer patients, and promising the power of AI-assisted pathology.
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.09 - Pathology (ID 173)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Pathology
- Presentations: 1
- Moderators:
- Coordinates: 9/08/2019, 09:45 - 18:00, Exhibit Hall
-
+
P1.09-11 - Influences of Sampling Method to Morphological Feature Measurement of Lung Cancer Cell (ID 886)
09:45 - 18:00 | Author(s): Noriko Motoi
- Abstract
Background
Progress of imaging technologies in the field of histopathology enables us to exploit artificial intelligence (AI) techniques to detect cancer based on digital images for screening or quality assurance of diagnosis process. Nowadays, reports on the application of AI to cancer detection which claim 99-percent detection accuracy are found in every proceedings or journal of digital pathology. However, little attention has been paid to the influences of sampling method to AI-based histological diagnosis.
Method
Whole slide images of hematoxylin and eosin (H&E) stained slides collected from 94 non-small cell lung cancer (NSCLC) cases were captured by a virtual slide scanner (NanoZoomer, Hamamatsu Photonics, Japan). Sampling methods were needle biopsy (59 cases), operation (12 cases) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (29 cases). Regions of interest (ROI) were selected by an experienced pathologist. After selecting tumor cells only by AI-based tumor cell detecter (Figure.1), following morphological features were calculated: nuclear area, perimeter (Peri), circularity (Circ) and five texture features, i.e., angular secondary moment (ASM), contrast(Cont), homogeneity (Hom) and entropy (Ent) of gray level co-occurrence matrix (GLCM), and contour complexity (CC).
Result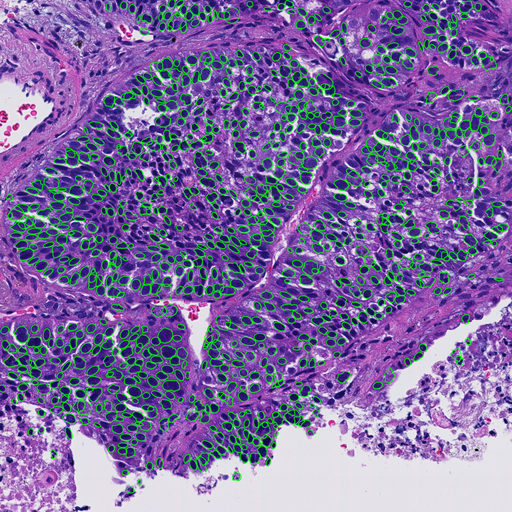
We found significant differences (p<0.05) in most of feature values except nuclear area and perimeter.
Conclusion
Our results suggest that methods of sampling significantly affect morphological feature values of nucleus and this fact must be taken into consideration when applying AI-based techniques to tissue image classification.
-
+
P2.09 - Pathology (ID 174)
- Event: WCLC 2019
- Type: Poster Viewing in the Exhibit Hall
- Track: Pathology
- Presentations: 2
- Now Available
- Moderators:
- Coordinates: 9/09/2019, 10:15 - 18:15, Exhibit Hall
-
+
P2.09-10 - INSM1 Is a Good Marker for Diagnosis of Small Cell Lung Carcinoma Even When Neuroendocrine Marker Negative (Now Available) (ID 1104)
10:15 - 18:15 | Author(s): Noriko Motoi
- Abstract
Background
To diagnose small cell lung carcinoma (SCLC), neuroendocrine (NE) phenotype markers such as chromogranin A, synaptophysin and CD56 are helpful. However, because they are dispensable, SCLCs occur without neuroendocrine phenotypes. Insulinoma-associated protein 1 (INSM1) is a transcription factor for neuroendocrine differentiation and has emerged as a single practical marker for SCLC.
Method
Using the surgical samples of 141 NE tumors (78 SCLCs, 44 large cell neuroendocrine carcinomas (LCNECs), and 19 carcinoids), and 246 non-NE carcinomas, we examined the immunohistochemical expression and prognostic relevance of INSM1 in association with NE phenotype markers in each histologic type. We evaluated its sensitivity and specificity for SCLC diagnosis, as well as its usefulness to diagnose SCLC without NE marker expression and to estimate the prognosis of the subgroups of SCLC stratified by the expression levels of the NE markers. Those of 13 lung cancer cell lines (9 SCLCs and 4 ADCs) were also evaluated.
Result
INSM1 was expressed in SCLCs (92%, 72/78), LCNECs (68%, 30/44), and carcinoids (95%, 18/19). Additionally, among SCLCs with no expression of NE phenotype markers (n=12), 9 (75%) were positive for INSM1. These data suggest the superiority of INSM1 to the phenotype markers. SCLC with low INSM1 expression (n=28) had a significantly better prognosis (P=0.040) than the high-INSM1 group (n=50). Only 7% of adenocarcinomas (9/134) and 4% of squamous cell carcinomas (4/112) were positive for INSM1. In cell lines, most SCLCs were positive for INSM1 (7/9), whereas all ADCs were negative (0/4).
Conclusion
Our study revealed that INSM1 is highly sensitive to detect SCLC, is positive in most phenotype marker-negative SCLCs and can estimate prognosis. INSM1 will be a promising marker for SCLC.
-
+
P2.09-24 - IASLC Global Survey for Pathologists on PD-L1 Testing for Non-Small Cell Lung Cancer (ID 906)
10:15 - 18:15 | Author(s): Noriko Motoi
- Abstract
Background
PD-L1 immunohistochemistry (IHC) is now performed for advanced non-small cell lung cancer (NSCLC) patients to examine their eligibility for pembrolizumab treatment, as well as in Europe for durvalumab therapy after chemoradiation for stage III NSCLC patients. Four PD-L1 clinical trial validated assays (commercial assays) have been FDA/EMA approved or are in vitro diagnostic tests in multiple countries, but high running costs have limited their use; thus, many laboratories utilize laboratory-developed tests (LDTs). Overall, the PD-L1 testing seems to be diversely implemented across different countries as well as across different laboratories.
Method
The Immune biomarker working group of the IASLC international pathology panel conducted an international online survey for pathologists on PD-L1 IHC testing for NSCLC patients from 2/1/2019 to 5/31/2019. The goal of the survey was to assess the current prevalence and practice of the PD-L1 testing and to identify issues to improve the practice globally. The survey included more than 20 questions on pre-analytical, analytical and post-analytical aspects of the PDL1 IHC testing, including the availability/type of PD-L1 IHC assay(s) as well as the attendance at a training course(s) and participation in a quality assurance program(s).
Result
344 pathologists from 310 institutions in 64 countries participated in the survey. Of those, 38% were from Europe (France 13%), 23% from North America (US 17%) and 17% from Asia. 53% practice thoracic pathology and 36%, cytopathology. 11 pathologists from 10 countries do not perform PD-L1 IHC and 7.6% send out to outside facility. Cell blocks are used by 75% of the participants and cytology smear by 9.9% along with biopsies and surgical specimens. Pre-analytical conditions are not recorded in 45% of the institutions. Clone 22C3 is the most frequently used (61.5%) (59% with the commercial assay; 41% with LDT) followed by clone SP263 (45%) (71% with the commercial assay; 29% with LDT). Overall, one or several LDTs are used by 57% of the participants. A half of the participants reported turnaround time as 2 days or less, while 13% reported it as 5 days or more. Importantly, 20% of the participants reported no quality assessment, 15%, no formal training session for PD-L1interpretation and 14%, no standardized reporting system.
Conclusion
There is marked heterogeneity in PD-L1 testing practice across individual laboratories. In addition, the significant minority reported a lack of quality assurance, formal training and/or standardized reporting system that need to be established to improve the PD-L1 testing practice globally.


