Virtual Library
Start Your Search
Gwo-Fuang Ho
Author of
-
+
P3.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 981)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.15-21 - Real-World Experience of First-Line Afatinib Treatment in Patients with EGFR Mutant Advanced Non-Small Cell Lung Cancer (ID 13600)
12:00 - 13:30 | Author(s): Gwo-Fuang Ho
- Abstract
Background
Published reports of first-line afatinib treatment efficacy, side-effects and resistance mechanism in the real-world setting are lacking.
a9ded1e5ce5d75814730bb4caaf49419 Method
A retrospective observational study of patients with EGFR mutant advanced non-small cell lung cancer (NSCLC) receiving first-line afatinib in University Malaya Medical Center from 1st December 2014 to 30th April 2018.
4c3880bb027f159e801041b1021e88e8 Result
Twenty-two of 33 patients on first-line afatinib were eligible for analysis. The patients’ demographic and clinical characteristics are as shown in Table 1. The mPFS was 14.3 months, overall response rate was 86.3% (19/22) and disease control rate was 95.5% (21/22). The median time-to-treatment failure was 16.2 months. The median overall survival has not been reached but 12-month survival rate was 81.8% (18/22).
A patient with exon 18 G719X and exon 20 S768I mutation had received treatment for 23.3 months without disease progression (PD). The PFS of a patient with exon 20 insertion was 9 months and of another patient with exon 18 G719X and exon 20 T790M mutations was 4.4 months. Of patients with brain metastases, the PFS of 2 patients treated with stereotactic radiosurgery (SRS) was 15.9 months and 9 months, respectively while that of a patient who had whole brain radiotherapy (WBRT) and a patient who underwent debulking surgery only was 16.5 months and 2.6 months, respectively.
The incidence of side-effects was rash 90.1% (20/22), stomatitis 63.3% (14/22), paronychia 72.7% (16/22), and diarrhea 77.3% (17/22). One (4.5%) patient each had grade 3 diarrhea and cutaneous lesions. Of 17 patients with PD, 2 (11.8%) had PD in the brain. T790M mutation was detected in 62.2% (8/13) patients who underwent repeat biopsy.
8eea62084ca7e541d918e823422bd82e Conclusion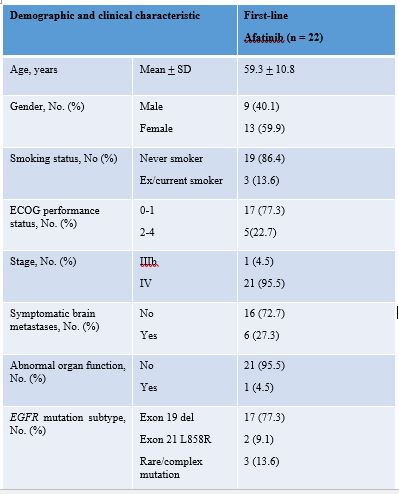
Afatinib is an effective treatment of EGFR-mutant advanced NSCLC. When combined with SRS or WBRT, afatinib conferred good PFS in patients with symptomatic brain metastases. Severe side effects are uncommon and T790M mutation was the commonest resistance mechanism.
6f8b794f3246b0c1e1780bb4d4d5dc53


