Virtual Library
Start Your Search
Junling Li
Author of
-
+
P3.13 - Targeted Therapy (Not CME Accredited Session) (ID 979)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.13-17 - A Retrospective Study: Central Nervous System Response to Osimertinib in Patients with Advanced NSCLC (ID 11937)
12:00 - 13:30 | Author(s): Junling Li
- Abstract
Background
Central nervous system (CNS) metastases are common in patients with non-small-cell lung cancer (NSCLC). More than 30% of patients who progress during or after treatment with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have CNS metastases. Osimertinib, a third-generation EGFR-TKI, has been demonstrated promising intracranial efficacy in patients with advanced NSCLC from several large scale randomized control trials. We aimed to explore clinical impact of osimertinib for patients with CNS metastases, advanced NSCLC in real world setting.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with advanced NSCLC who received osimertinib after progression of prior EGFR-TKIs and CNS metastases on baseline brain scan were retrospectively collected from Cancer Hospital Chinese Academy of Medical Sciences. Primary outcome was objective response rate (ORR) and secondary objectives were disease control rate (DCR), progression-free survival (PFS), time to tumor response, median best percentage change from baseline in CNS target lesion (TL) size and safety.
4c3880bb027f159e801041b1021e88e8 Result
Between Apr 1, 2017, and Dec 30, 2017, 22 patients met selection criteria, 15 with ≥1 measurable CNS lesion (RECIST 1.1) were included in CNS evaluable for response (cEFR) set. The median duration of follow-up was 6.5 months. For overall 22 patients, ORR and DCR were 40.9% and 86.4%, respectively, with median PFS of 8.5 months (95% CI 4.1, 13.0). Of 15 patients in cEFR set, CNS ORR was 53.3% with complete responses reported in 3 patients (20.0%). Median best percentage change from baseline in CNS TL size was -40% (range: -100% ~ +60%) and median time to CNS tumor response was 1.3 months. CNS DCR was 80.0%. Median CNS PFS was not reached. Safety profile was acceptable and no new unexpected findings were found.
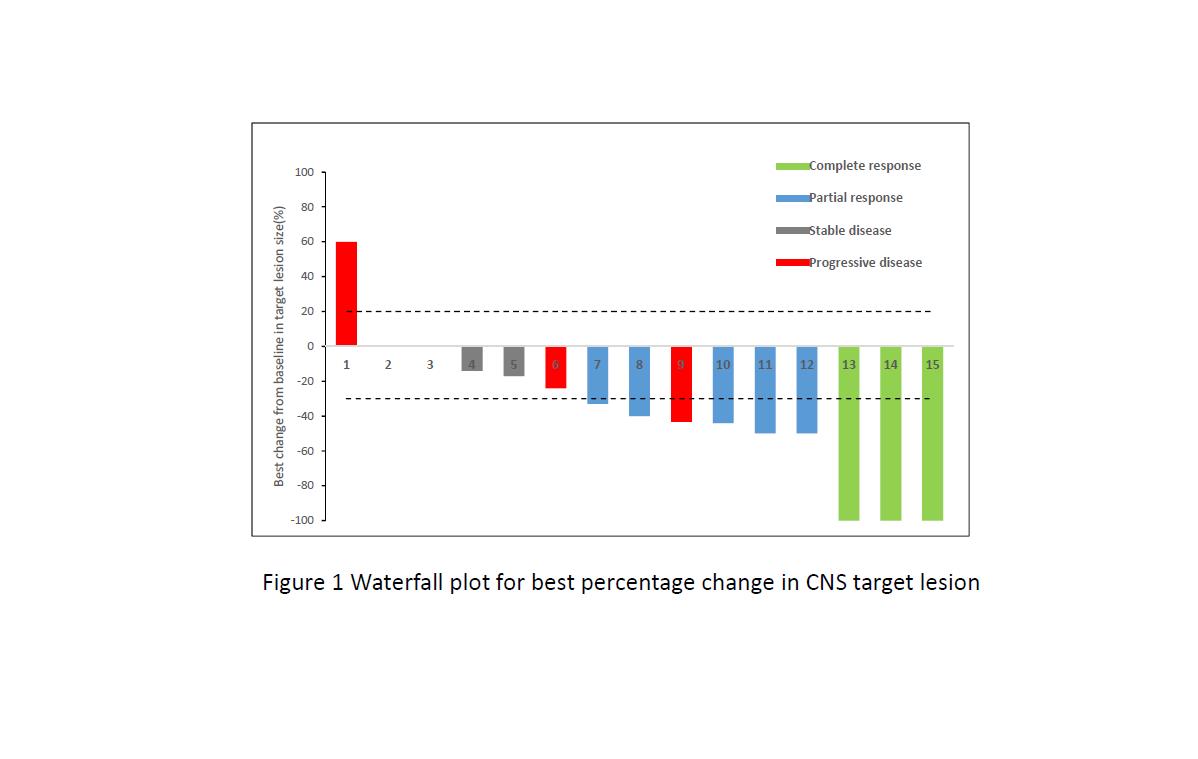
8eea62084ca7e541d918e823422bd82e Conclusion
This real world analysis further confirmed that osimertinib was indeed demonstrated clinically meaningful efficacy against CNS metastases in Chinese patients with advanced NSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53


