Virtual Library
Start Your Search
Preet Walia
Author of
-
+
P3.04 - Immunooncology (Not CME Accredited Session) (ID 970)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.04-20 - Correlation of Immune-Related Adverse Events and Response from Immune Checkpoint Inhibitors in Patients with Advanced NSCLC (ID 14138)
12:00 - 13:30 | Author(s): Preet Walia
- Abstract
Background
Immune checkpoint inhibitors (ICIs) are associated with a unique set of toxicities termed immune-related adverse events (irAEs). The association between response to ICI therapy and development of irAEs has been documented in various cancer types.
a9ded1e5ce5d75814730bb4caaf49419 Method
Stage IV non-small cell lung cancer (NSCLC) patients treated with ICIs at the Princess Margaret Cancer Centre between 2013 and 2016 were followed for treatment response, treatment duration, survival, and toxicity. The relationship between treatment outcomes and occurrence of irAEs was examined.
4c3880bb027f159e801041b1021e88e8 Result
A total of 97 advanced NSCLC patients were followed. Most patients (81%) received anti-PD-1 agents, 17% received anti-PD-L1 agents, and 2% received combination anti-PD-L1 plus anti-CTLA-4 therapy. Median follow up for the cohort was 5.1 months (0.3-38.1 months) from treatment start. Demographic and tumour characteristics were balanced between the groups. IrAEs occurred in half of patients (51%) on ICIs and grade≥3 irAEs in 7%. The most commonly observed irAEs were arthralgia (13%), diarrhea/colitis (12%), and skin rash (11%). Discontinuation of treatment due to irAEs occurred in 10% of patients, half of whom experienced grade≥3 irAEs.
The overall response rate to ICIs was 23%, with the majority occurring by week 8 of treatment (16/22). Response was non-evaluable in one patient and this was excluded from response analysis. Patients with grade≥3 irAEs were more likely to have response to treatment compared to those with grade I/II irAEs and no irAEs (67% vs 17% vs 23%, p=0.035), Table 1. Smoking status was not associated with response rate or frequency of irAEs. Median survival was not reached in those with grade≥3 irAEs, 15.7 months in those with grade I/II irAEs, and 7.4 months in those with no irAE (p=0.16). Duration of treatment did not differ significantly among the groups.
8eea62084ca7e541d918e823422bd82e Conclusion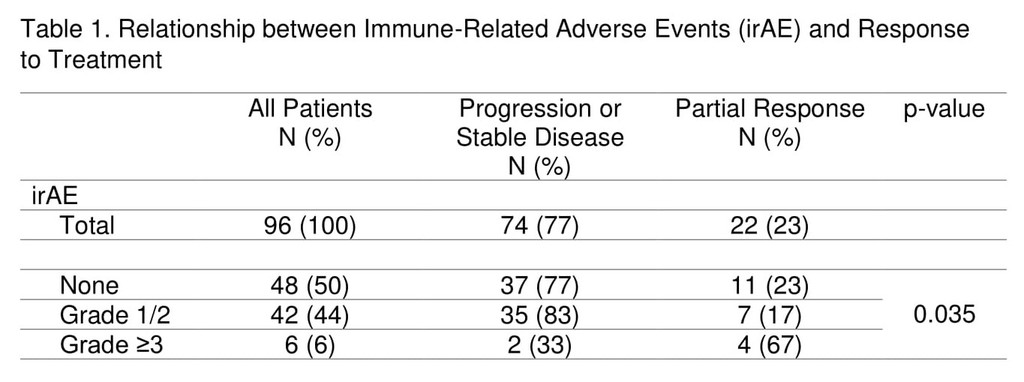
The occurrence of grade≥3 irAEs may be associated with treatment response in advanced NSCLC patients undergoing ICI therapy.
6f8b794f3246b0c1e1780bb4d4d5dc53


