Virtual Library
Start Your Search
Monika Olejniczak
Author of
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-17 - Long Survivors with Advanced Lung Cancer - Efficacy and Tolerability of Nivolumab (ID 11922)
12:00 - 13:30 | Author(s): Monika Olejniczak
- Abstract
Background
The group of the patients with advanced lung cancer surviving more than two years was limitated, particullary at era of the classic chemotherapy.In our material presented in 2013 on WCLC it made up 11%.The differences in efficacy betweeen Nivolumab and conventional treatment in pretreated patients remains unclear, particullary in the group with good response on chemotherapy with long interval between lines. We analyzed the efficacy and tolerance of Nivolumab in the long surviving group.
a9ded1e5ce5d75814730bb4caaf49419 Method
7 patients characterized by long earlier history of lung cancer were found. Baseline characteristic and historical treatment data are presented in table 1.
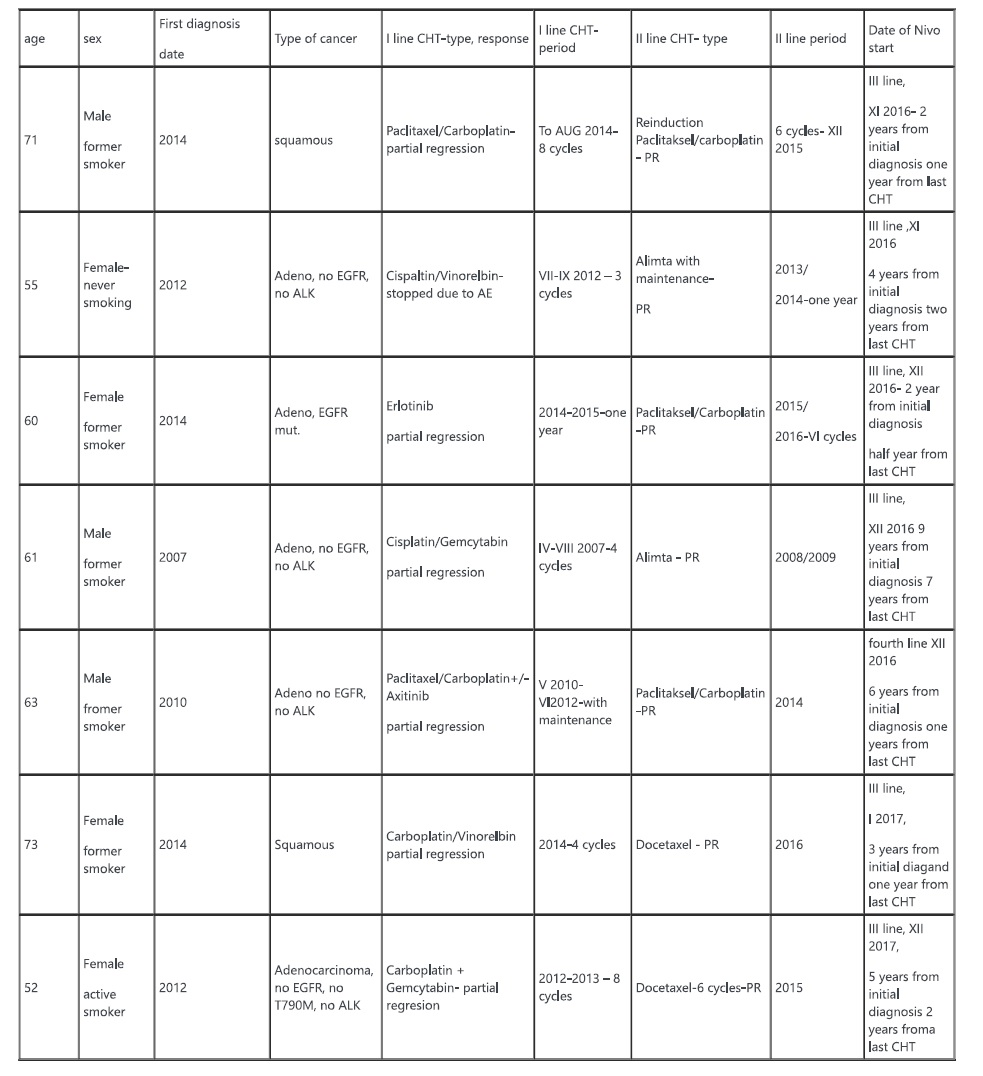
4c3880bb027f159e801041b1021e88e8 Result
Table nr 2 presents the efficacy and tolerability of Nivolumab.
The patients obtained 190 injections of drug and only one patient requires 2,5 months break beacuse of typical AE-tyreoiditis G3. Other short breaks were dependent on personal requests ,not from AE. Treatment time is 12-17 months and is plannig to continue .No progression has been noticed. Follow-up is planned.

8eea62084ca7e541d918e823422bd82e Conclusion
Long surviving patients with significant interval between chemotherapy lines show excellent tolerability and good efficacy of Nivolumab regardless of the type of previous chemotherapy.Efficacy and tolerance of these patients are comparable to described group with better prognosis from trials Checkmate 003 and 153
6f8b794f3246b0c1e1780bb4d4d5dc53


