Virtual Library
Start Your Search
Mike Sung
Author of
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-94 - Diagnostic Patterns of Non-Small Cell Lung Cancer at Princess Margaret Cancer Centre (ID 14178)
16:45 - 18:00 | Presenting Author(s): Mike Sung
- Abstract
Background
Accurate classification of lung cancer subtypes has become critical in tailoring lung cancer treatment. Our study aimed to evaluate changes in diagnostic testing and pathologic subtyping of advanced non-small cell lung cancer (NSCLC) over time at a major cancer centre.
a9ded1e5ce5d75814730bb4caaf49419 Method
A review of patients diagnosed with advanced NSCLC at the Princess Margaret Cancer Centre between 2007-2009 and 2013-2015 was performed. Diagnostic method, sample type and site, pathologic subtype, and use of immunohistochemical (IHC) staining and molecular testing were abstracted.
4c3880bb027f159e801041b1021e88e8 Result
A total of 238 patients were reviewed in 2007-2009 and 283 patients in 2013-2015 (Table 1). Over time, the proportion of patients diagnosed with adenocarcinoma increased from 60.9% to 73.1% while NSCLC-not otherwise specified (NOS) diagnosis decreased from 18.9% to 6.4%, p<0.0001. There was a decrease in use of diagnostic bronchoscopy (26.9% vs 18.4%) and an increase with mediastinal sampling procedures including endobronchial ultrasound (9.2% vs 20.5%), p=0.0001. A substantial reduction in cases reported as NSCLC-NOS was observed among bronchoscopy, image-guided, and mediastinal sampling procedures. The reduction in NSCLC-NOS was also predominantly seen in cytology samples, from 22.0% to 4.0% (p<0.0001).
IHC use increased over time from 41.6% to 76.3% (p<0.0001). Patients with larger samples and IHC analysis were more likely to have biomarker testing performed (both p<0.01). Within the group diagnosed with NSCLC-NOS, the use of IHC increased non-significantly from 64% (29/45) to 94% (16/18). With the exception of bronchoscopy samples, use of IHC increased significantly with each method of diagnosis and sample type.
8eea62084ca7e541d918e823422bd82e Conclusion
Customizing treatment based on pathologic subtype and molecular genotype has become key in treating advanced lung cancer patients. Greater accuracy of pathologic diagnosis is being achieved including through use of routine IHC.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.04 - Immunooncology (Not CME Accredited Session) (ID 970)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.04-20 - Correlation of Immune-Related Adverse Events and Response from Immune Checkpoint Inhibitors in Patients with Advanced NSCLC (ID 14138)
12:00 - 13:30 | Presenting Author(s): Mike Sung
- Abstract
Background
Immune checkpoint inhibitors (ICIs) are associated with a unique set of toxicities termed immune-related adverse events (irAEs). The association between response to ICI therapy and development of irAEs has been documented in various cancer types.
a9ded1e5ce5d75814730bb4caaf49419 Method
Stage IV non-small cell lung cancer (NSCLC) patients treated with ICIs at the Princess Margaret Cancer Centre between 2013 and 2016 were followed for treatment response, treatment duration, survival, and toxicity. The relationship between treatment outcomes and occurrence of irAEs was examined.
4c3880bb027f159e801041b1021e88e8 Result
A total of 97 advanced NSCLC patients were followed. Most patients (81%) received anti-PD-1 agents, 17% received anti-PD-L1 agents, and 2% received combination anti-PD-L1 plus anti-CTLA-4 therapy. Median follow up for the cohort was 5.1 months (0.3-38.1 months) from treatment start. Demographic and tumour characteristics were balanced between the groups. IrAEs occurred in half of patients (51%) on ICIs and grade≥3 irAEs in 7%. The most commonly observed irAEs were arthralgia (13%), diarrhea/colitis (12%), and skin rash (11%). Discontinuation of treatment due to irAEs occurred in 10% of patients, half of whom experienced grade≥3 irAEs.
The overall response rate to ICIs was 23%, with the majority occurring by week 8 of treatment (16/22). Response was non-evaluable in one patient and this was excluded from response analysis. Patients with grade≥3 irAEs were more likely to have response to treatment compared to those with grade I/II irAEs and no irAEs (67% vs 17% vs 23%, p=0.035), Table 1. Smoking status was not associated with response rate or frequency of irAEs. Median survival was not reached in those with grade≥3 irAEs, 15.7 months in those with grade I/II irAEs, and 7.4 months in those with no irAE (p=0.16). Duration of treatment did not differ significantly among the groups.
8eea62084ca7e541d918e823422bd82e Conclusion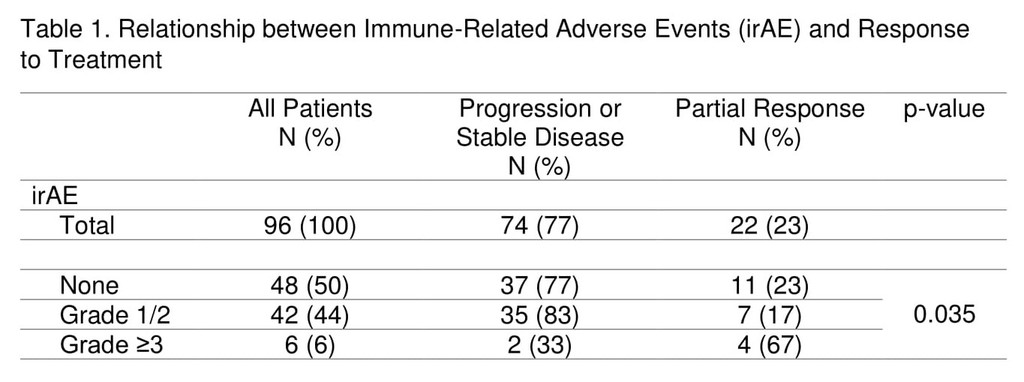
The occurrence of grade≥3 irAEs may be associated with treatment response in advanced NSCLC patients undergoing ICI therapy.
6f8b794f3246b0c1e1780bb4d4d5dc53


