Virtual Library
Start Your Search
Qiuling Shi
Author of
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-87 - Profiling the Symptom Burden of Patients with Metastatic NSCLC Receiving Either Chemotherapy or Targeted Therapy: Real-World Data (ID 13348)
16:45 - 18:00 | Presenting Author(s): Qiuling Shi
- Abstract
Background
An understanding of the patient experience is lacking for newly developed cancer treatments, such as targeted therapies. We profiled the patient-reported outcome (PRO)-measured symptom burden experienced by patients with metastatic non-small cell lung cancer (mNSCLC) during 6 months of conventional chemotherapy or targeted therapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
During 2017, patients with mNSCLC at a single institution were recruited and completed the MD Anderson Symptom Inventory lung cancer module (MDASI-LC) at clinic visits. The MDASI-LC assesses the severity of 13 core and 3 lung-cancer-specific symptoms and 6 interference items on 0‒10 scales (0=no symptom or interference, 10=worst imaginable symptom or complete interference). Descriptive statistics for MDASI-LC scores over 6 months of treatment were summarized. Symptom trajectories for the chemotherapy patients versus the targeted-therapy patients were compared via linear mixed-effects models.
4c3880bb027f159e801041b1021e88e8 Result
Of 65 patients receiving chemotherapy and 27 receiving targeted therapy, the targeted-therapy group had more women (74% vs. 49%, P=0.029) and younger patients (57.6±12.2 vs. 64.2±9.9 years, P=0.012). Before treatment, both groups reported similar symptom burden, although sadness was worse in the targeted-therapy group (2.4±1.6 vs. 0.8±1.5, P=0.021). During the first 60 days of treatment, patients receiving chemotherapy reported significant increase in pain (estimate (est)=0.03, P=0.037) and interference with walking (est=0.04, P=0.025). Compared with those receiving chemotherapy, patients receiving targeted therapy experienced significantly less severe pain (est=‒1.17, P=0.024), fatigue (est=‒1.16, P=0.019), and shortness of breath (est=‒1.23, P=0.028) and less interference with walking (est=‒1.23, P=0.042) (figure 1). More severe dry mouth was reported by patients undergoing targeted therapy (est=1.17, P=0.027).
8eea62084ca7e541d918e823422bd82e Conclusion
This real-world data demonstrates that, compared with conventional chemotherapy, targeted therapy correlates with less impairment of physiological condition and functioning in patients with mNSCLC. Additional follow up will confirm and expand these findings about the patient experience relative to treatment response.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-109 - Real-World Patient-Reported Outcome Assessment of Patients with Metastatic Non-Small Cell Lung Cancer (ID 12213)
12:00 - 13:30 | Author(s): Qiuling Shi
- Abstract
Background
Patient-Reported Outcomes (PROs) provide information on patient treatment experience. We have established a real-world Advanced Non-Small Cell Lung Holistic Registry (ANCHoR) to understand how the advent of immunotherapy impacts treatment choice, clinical outcomes, and PROs of metastatic non-small cell lung cancer (mNSCLC). The aim of this analysis is to report early results of baseline symptom status and quality of life among mNSCLC patients using the MD Anderson Symptom Inventory lung cancer module (MDASI-LC) and EuroQol-5D 5-level version (EQ-5D-5L).
a9ded1e5ce5d75814730bb4caaf49419 Method
During 2017, patients with mNSCLC at a single institution were enrolled in ANCHoR and completed the PRO questionnaires at clinic visits. MDASI-LC consists of thirteen core and three lung cancer-specific symptom severity questions, and six interference items rated on 0-10 scales (0 = no symptom or interference, 10 = worst imaginable symptom or complete interference). EQ-5D-5L captures five health state dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression rated on a five-level scale (1= no problems, 5= extreme problems). A single visual analogue scale (VAS) on EQ-5D-5L records patient self-rated health between ”best imaginable” (100) and “worst imaginable” (0) health state. Descriptive statistics for PRO scores at baseline are summarized.
4c3880bb027f159e801041b1021e88e8 Result
Forty-two patients completed baseline PROs before the start of therapy. Mean patient age was 63 years and 45% were males. For MDASI-LC, the mean scores for the core symptom, lung cancer-specific symptom, and interference subscales at baseline were 2.2 (standard deviation [SD] = 2.80), 2.1 (SD = 2.80), and 2.8 (SD = 3.10), respectively. Fatigue was the most severe symptom reported at baseline (mean = 4.1, SD = 3.01), followed by shortness of breath (mean = 3.2, SD = 2.81) and pain (mean = 3.19, SD = 3.00). The highest percentages of patients reporting moderate to severe symptom levels (score of ≥5) were 38% for fatigue, 33% for pain, 31% for drowsiness, 29% for shortness of breath and disturbed sleep, and 26% coughing. For EQ-5D-5L, 91% of patient reported problems with self-care, 81% with mobility, 48% with usual activity and anxiety, and 33% with pain. Mean EQ-5D VAS was 73.9 (SD = 18.2).
8eea62084ca7e541d918e823422bd82e Conclusion
Prior to the start of treatment, fatigue, pain, drowsiness, disturbed sleep, and coughing were the most common symptoms with fatigue, shortness of breath, and pain being the most severe. Additional follow up will confirm and expand these findings and will also allow us to examine change in PROs after first-line treatment is administered.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 981)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.15-08 - Patient-Reported Outcomes (PROs) in Patients with Lung Resection: Open Versus Minimal Invasive Surgery (ID 13291)
12:00 - 13:30 | Presenting Author(s): Qiuling Shi
- Abstract
Background
As a novel and cutting-edge indicator to evaluate the quality of surgery, e.g. minimal invasive surgery (MIS), Patient-Reported Outcomes (PROs) has not been implemented in patients with lung resection in the real world. This study aims to profile PRO-measured symptom burden in patients with lung resection of either open surgery (OS) or MIS.
a9ded1e5ce5d75814730bb4caaf49419 Method
We conducted a prospective study on lung resection patients from November 22, 2017 to April 23, 2018. MD Anderson Symptom Inventory-lung cancer module (MDASI-LC) was used to assess the severity of perioperative symptoms and how they interfered with daily functioning. MDASI-LC was administered on the pre-operation day and daily after surgery up to the day of discharge. Trajectories of symptom severity and interference were compared between OS and MIS via mixed effects models.
4c3880bb027f159e801041b1021e88e8 Result
Among 78 lung resection patients, 22 received OS and 56 received MIS. Most of the resection types were lobectomies (74.4%). All patients completed pre-operation assessment and the missing rate after surgery was less than 10%. There were no statistically significant differences between OS and MIS in pre-operation variables including age, BMI, hemoglobin, neutrophil ratio, platelets and creatinine. Within the first week after surgery, the most severe symptoms were pain, cough, fatigue, disturbed sleep and dry mouth for OS patients and pain, fatigue, cough, disturbed sleep and shortness of breath for MIS patients. MIS patients reported better physiological functioning (walking, general activity and work) than did OS patients in the first 7 days post surgery (p=0.013). Among patients with MIS, those with stage II-IV reported more severe fatigue than did stage I patients over the hospitalization (p=0.018).
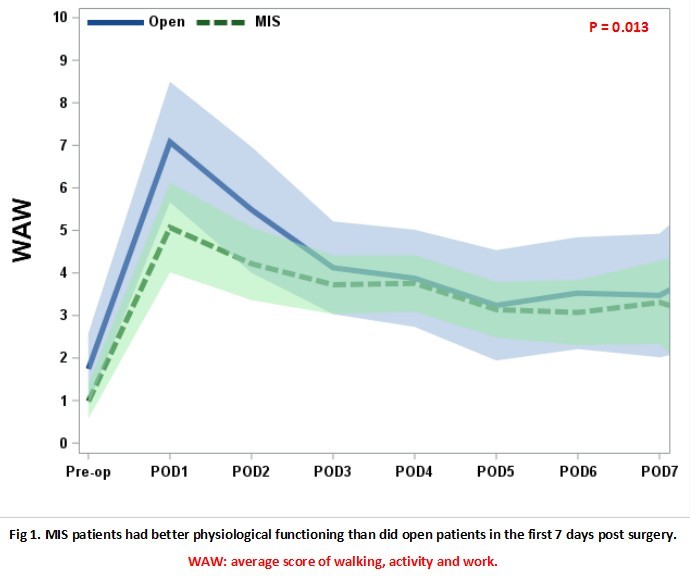
8eea62084ca7e541d918e823422bd82e Conclusion
This real world data preliminarily profile patients’ experiences over a short period after lung resection. Compared to OS, MIS did not show a significant advantage in symptom severity, but related to better functioning on daily living. Additional recruitments and follow-up will expand patients’ perceptions to the full trajectory of recovering from lung resection.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P3.15-29 - Defining the Symptom Burden of Non-Small Cell Lung Cancer (ID 12361)
12:00 - 13:30 | Author(s): Qiuling Shi
- Abstract
Background
Symptom burden is disease and treatment symptom severity and its impact on daily functioning. Symptom monitoring has demonstrated improved cancer patient outcomes, including quality of life, resource utilization, ability to continue treatment, and survival. The use of disease-specific patient-reported outcomes (PRO) measures facilitates individualized symptom monitoring and management. The purpose of this study was to describe symptom experience from the patient perspective and identify key symptoms for a PRO measure of non-small cell lung cancer (NSCLC) symptom burden.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with NSCLC described their symptom experience in single qualitative interviews. Content analysis was used to define the content for a PRO measure of NSCLC symptom burden.
4c3880bb027f159e801041b1021e88e8 Result
Mean age of the 40 patients interviewed was 66.1 years (standard deviation = 10.9); 60.0% were male, 77.5% were white, and 56.4% had stage IV disease. Content analysis found a total of 32 symptoms, 6 reported by ≥ 20% of participants (see Table 1). Symptoms varied based on treatment modality (chemotherapy versus radiation therapy), but not stage of disease. Numbness or tingling and sore mouth were described only by patients who had received chemotherapy. Patients volunteered ways in which symptoms impacted daily activities and relationships.
Table 1. Patient quotes from qualitative interviews describing the 6 most common symptoms (reported by ≥ 20% of participants) Symptom Participant Quote Shortness of breath “The heaviness, it’s like a—wow, I don’t know how to explain it—like a rock and hard to breathe sometimes, just shortness of breath. Of course, the more I try to walk, or whatever, I’m more short of breath.”
‒ 67-year-old femaleCough “I had a real bad cough. I think I actually even broke a couple of ribs coughing so much.”
‒ 52-year-old male
Distress “Terrifying. There’s no ways about it. You know, it’s a terrifying experience, especially when it’s dropped in your lap and you have to deal with it. You go through a lot physically and mentally.”
‒ 67-year-old maleFatigue “I'm more tired. I take a lot of naps where I never had been a nap person. Before I had all my energy, and I was doing lots of things, and now I'm wore out. I wake up, and I'm wore out.”
‒ 53-year-old malePain “You keep trying to move it to make it feel better and no matter where you put it, it doesn’t feel any better … most of the time it will bother me after I get out of bed in the morning for a while. And then if I go try to take a nap, I’ll go ahead and take something for pain because I can’t lay there and—I just keep moving it and moving it and nothing helps.”
- 68-year-old maleConstipation “I didn’t have a bowel movement. I had always taken the stool softeners because they told me to do that. And I kept thinking, “Well, it’s going to work. It’s going to work.” Finally, I was in so much pain that I couldn’t stand anymore, so I went to the hospital … and they ended up physically removing, which was horrible.”
‒ 68-year-old female
8eea62084ca7e541d918e823422bd82e Conclusion
Patients with NSCLC experience numerous symptoms related to disease and treatment. Shortness of breath, cough, distress, fatigue, pain, and constipation were commonly reported symptoms, suggesting that clinicians should routinely and proactively monitor the presence and severity of these symptoms in NSCLC clinical care. In patients receiving chemotherapy, attention to specific treatment-related symptoms, including symptoms of neuropathy and sore mouth, is needed. While stage of disease does not produce unique symptoms, the severity of the symptoms may possibly vary by stage of disease. Clinicians should also be aware that symptoms result in interference with daily activities, relationships, life plans, treatment adherence, and mood.
6f8b794f3246b0c1e1780bb4d4d5dc53


