Virtual Library
Start Your Search
James Paul
Author of
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-43 - ADSCaN: A Randomised Phase II Study of Accelerated, Dose Escalated, Sequential Chemo-Radiotherapy in Non-Small Cell Lung Cancer (NSCLC) (ID 13934)
16:45 - 18:00 | Author(s): James Paul
- Abstract
Background
Lung cancer is the most common cause of cancer mortality in the UK, and NSCLC accounts for approximately 85% of all lung cancers. Most patients present with inoperable disease therefore radiotherapy plays a major role in treatment. However, the majority of patients are not suitable for gold standard treatment (concurrent chemo-radiotherapy) due to performance status and comorbidities. Novel strategies integrating radiotherapy advances and radiobiological knowledge need to be evaluated in patients treated with sequential chemo-radiotherapy. Four separate accelerated dose escalated radiotherapy schedules have been completed in UK (CHART-ED{1}, IDEAL-CRT{2}, I-START{3} and Isotoxic IMRT{4}). ADSCaN will compare these schedules with a UK standard sequential chemo-radiotherapy schedule. A combined randomized phase II screening / ‘pick the winner’ approach will identify the best schedule to take into a randomised phase III study against conventionally fractionated radiotherapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Suitable patients will have histologically / cytologically confirmed, stage III NSCLC and be able to undergo chemo-radiotherapy treatment. The study will recruit 360 patients; 130 on the standard arm and 60 on each experimental arm. Patients will complete 2-4 cycles of platinum based chemotherapy before being randomised to one of the radiotherapy schedules.
Logistic / capacity challenges make it impractical for sites to open all experimental trial arms; a novel trial design allows centres to select upfront the experimental arms they are able to participate in and all will offer the standard arm.
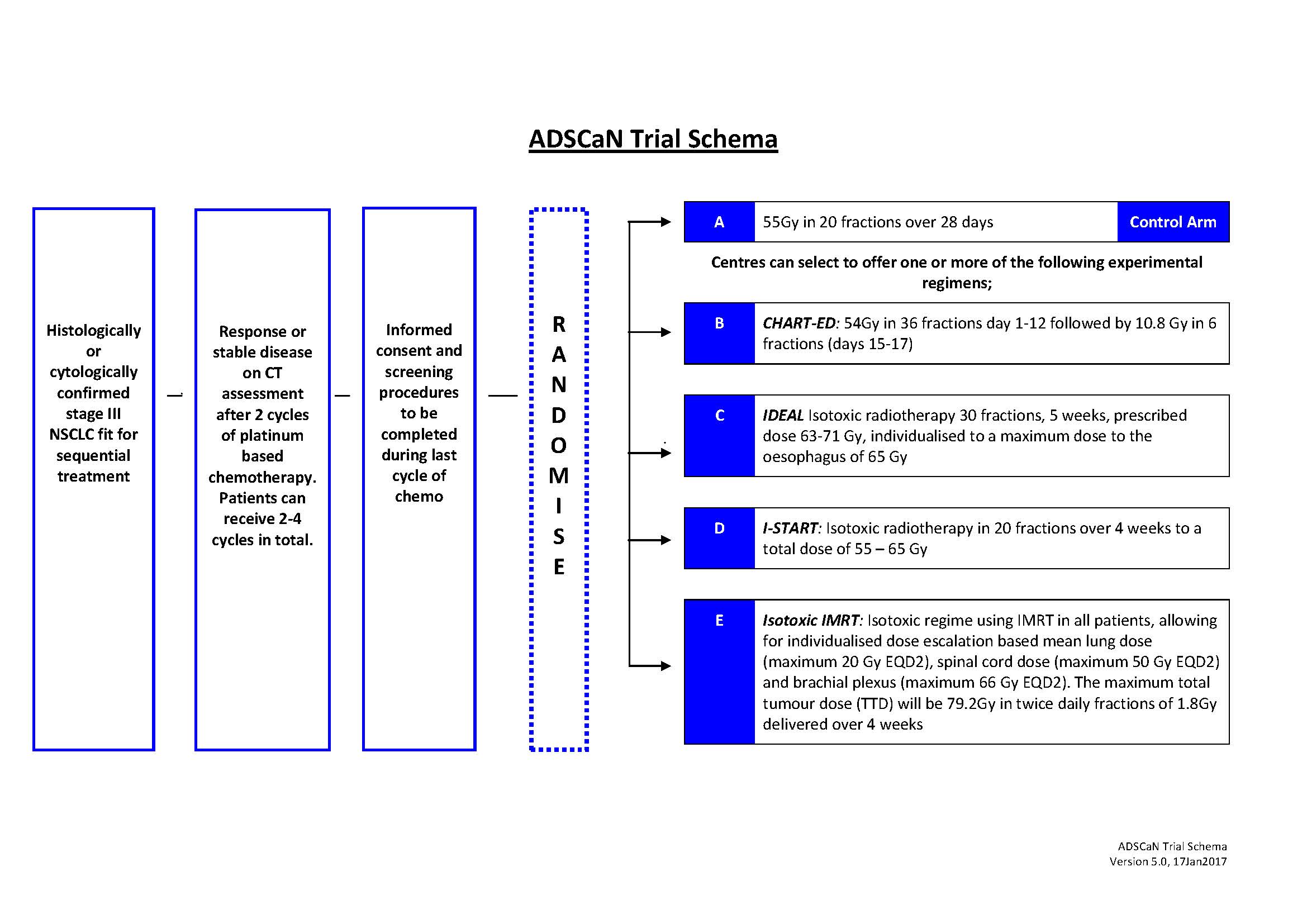
4c3880bb027f159e801041b1021e88e8 Result
CURRENT STATUS
CRUK is funding this multicentre study which is being co-ordinated by the CRUK CTU Glasgow. The study opened to recruitment on 22/08/2017 with planned recruitment lasting 3 years 8 months. The study includes a tailored QA programme through the UK RTTQA Group. 20 of the 36 sites expressing interest have started the QA process, 12 have completed with a further 8 expected to complete in the next few months.
8eea62084ca7e541d918e823422bd82e Conclusion
Section Not Applicable
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.17 - Treatment of Locoregional Disease - NSCLC (Not CME Accredited Session) (ID 966)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.17-02 - Cardiopulmonary Exercise Tests in Lung Cancer Patients Treated Radical Radiotherapy and Chemotherapy – Feasibility Study (ID 14145)
16:45 - 18:00 | Author(s): James Paul
- Abstract
Background
Cardiopulmonary exercise testing (CPET) is based on the principle that system failure can be detected when the system is under stress. CPET allows measurement of peak oxygen consumption (peak VO2), the gold standard measure of exercise performance, and can identify the causes of exercise limitation. Variables measured at CPET can predict mortality in various disease states and is used to assess fitness for surgery. Lung cancer patients often have pre-existing cardiopulmonary disease, however there is limited data on the role of CPET in patients treated with radical radiotherapy (RRT). Recent RTOG 0617 study reported worse survival with RRT dose escalation, which was attributed to cardiopulmonary toxicity. This study aimed to investigate the feasibility of using CPET to study the effects of RRT on exercise capacity and to assess its cardiac and pulmonary components.
a9ded1e5ce5d75814730bb4caaf49419 Method
During the period 2003 – 2009, NSCLC patients undergoing RRT consented to participate in this prospective study. Alongside standard incremental CPETs, patients were assessed with pulmonary function tests and standardized measure of activity of daily living, the London Chest Activity of Daily Living scale (LCADL) at four different time points: pre-RRT and at 6 weeks, 6 months and 12 months post RRT.
4c3880bb027f159e801041b1021e88e8 Result
Thirty-eight patients participated. Median age was 66 years, and Karnofsky Performance Status >70. Using TNM 5th Edition, staging was T1-2/T3-4 = 50%/50%, N0-X/N1-2 = 50%/50% and M0/1 = 92%/8%. Planned RRT was completed by 34 pts.
Over the 12 months the peak VO2 (l/min) decreased (p overall = 0.009) from a median 0.83 by a maximum of 0.12 at 6 m, thus demonstrating a decline in exercise performance.
The VE/CO2AT increased (p overall = 0.005) post RT from a median 40, most clearly at 3 months (5, p=.028). Furthermore peak alveolar-arterial (A-a) gradient increased (p overall =.001) from a baseline median value of 38.5 at 6w (3.15, p=.046) and 3m (5.75, p=.013) respectively. These results indicate a decline in ventilatory efficiency following RRT.
No significant changes were seen in oxygen pulse, a surrogate measure of cardiac function.
LCADL completion reduced after 6w. The median baseline score was 22 and a statistically significant difference could not be detected over time (p overall = 0.502)
8eea62084ca7e541d918e823422bd82e Conclusion
The results indicate that CPET is able to detect a decline in exercise performance after RRT and that in this study the decline appears to be driven by a reduction in respiratory function. These results require confirmation in a larger study.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.17-22 - Cardiac Biomarkers in CART Study (CARdiac Toxicity in Lung Cancer Patients After Chemo-Radiotherapy). (ID 14165)
16:45 - 18:00 | Author(s): James Paul
- Abstract
Background
Lung cancer has the highest incidence and mortality. Chemo-radiation (CRT) can achieve curative outcomes, but there is limited knowledge of the associated toxicity. The results of the RTOG 0617 study highlighted the impact of cardiac toxicity. Further investigation of cardiopulmonary toxicity is required.
a9ded1e5ce5d75814730bb4caaf49419 Method
This is a single centre, prospective observational study in which NSCLC patients undergoing RRT with or without chemotherapy were invited to participate. Evaluations include clinical assessment, cardiac MRI and ECG. Biomarker results are available for lattermost patients. Evaluations performed at baseline, during treatment, 6 weeks and 6 months after treatment.
4c3880bb027f159e801041b1021e88e8 Result
11 patients underwent translational blood sampling. Key details are given in table 1. Change in biomarker results is shown in figure 1. In this limited sample the change did not reach statistical significance. This study showed that 3/11 patients achieved hsTnT > 14 (suggestive of myocardial damage) during study period. 8/11 patients had NTproBNP > 125 (suggestive of increased heart failure risk) at some point on study. 3/11 demonstrated an NTproBNP levels > 400, a recognised cut off warranting cardiac echo.

Table 1 Patient
Baseline
Co-morbidities
Baseline ECG result
Treatment
Baseline NTproBNP, pg/ml
(maximum change post-baseline)
Baseline hsTnT, pg/ml
(maximum change post-baseline)
Hospital admission within 6 months (reason)
Status at 6 months
22
Normal
RRT
22
(+ 4%)
6.7
(+38%)
No
Alive
24
IHD, DM, COPD, RA
T-inversion, II, III, aVF
CRT
625
(+101%)
11.2
(+105%)
Yes (COPD, NSTEMI)
Alive
26
Normal
CRT
89
(+79%)
6.2
(+67%)
Yes (Chest pain)
Alive
27
CHOLES, HPT
Normal
RRT
81
(+80%)
5.5
(+47%)
Yes (Unknown)
Alive
28
HPT
Normal
RRT
32
(+106%)
7.1
(-20%)
No
Alive
29
IHD, DM, CHOLES
Sinus Bradycardia,
Ist Degree AV Block
RRT
Missing
(NA)
Missing
(NA)
No
Alive
30
COPD, HEPB
Sinus Bradycardia
CRT
113
(+148%)
7.6
(-16%)
No
Alive
31
AF
Normal
RRT
58
(+15%)
7.2
(+101%)
No
Alive
32
IHD, CHOLES, HPT
Normal
RRT
624
(+10%)
4.9
(+39%)
No
Alive
33
IHD, HPT
Normal
CRT
91
(+220%)
7.6
(+12%)
No
Alive
34
Normal
CRT
509
(-45%)
4.7
(-37%)
Yes (N+V, chest pain)
Dead
Table 1. Listing of comorbidities, treatment, hospital admissions, ECG results and baseline biomarkers. Abbreviations: RRT (radical radiotherapy), CRT (concurrent chemo-radiation), COPD (exacerbation of COPD), NSTEMI (non-elevated ST segment myocardial infraction), N+V (nausea and vomiting), IHD (ischaemic heart disease), DM (diabetes mellitus), HPT (hypertension), CHOLES (hypercholesterolemia), AF (atrial fibrillation), RA (rheumatoid arthritis), HEPB (Hepatitis B), NTproBNP(N-terminal pro-B-type natriuretic peptide), hsTnT (high sensitivity Troponin-T)
8eea62084ca7e541d918e823422bd82e Conclusion
Cardiac biomarkers and their changes indicate that further investigation may be required in some to exclude cardiac ischaemia and heart failure risks before or following treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53


