Virtual Library
Start Your Search
Sheenu Chandwani
Author of
-
+
P1.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 948)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.16-07 - Cost-Effectiveness of Pembrolizumab as 1st Line Treatment for Metastatic NSCLC Patients with High PD-L1 Expression in Singapore (ID 11719)
16:45 - 18:00 | Author(s): Sheenu Chandwani
- Abstract
Background
Pembrolizumab, an immune checkpoint inhibitor, has been approved as monotherapy for 1st line treatment of metastatic NSCLC with PD-L1 tumor proportion score (TPS) ≥50% based on the pivotal Keynote (KN)-024 study. This study aims to evaluate the cost-effectiveness of pembrolizumab compared with standard-of-care (SoC) platinum-based chemotherapy in patients with TPS≥50% from a societal perspective in Singapore based on results from KN024.
a9ded1e5ce5d75814730bb4caaf49419 Method
A known partitioned-survival model was adapted to estimate progression-free survival, overall survival, costs of treatments, adverse events and disease management, and health utilities over a time horizon of 20 years. The maximum treatment duration of 2 years was applied for pembrolizumab. Clinical and resource utilization inputs were based on data from KN024 study and input from local oncologists. Unit costs captured both patients’ payments and government subsidies. Utility scores in different health states were based on EQ-5D data from KN024 with weighting indices suggested by a local quality-of-life study. An annual discount rate of 3% was applied and a series of sensitivity and scenario analyses were conducted to address uncertainty.
4c3880bb027f159e801041b1021e88e8 Result
For 1st line treatment for NSCLC patients with TPS≥50%, pembrolizumab monotherapy is estimated to result in 0.91 quality-adjusted-life-years (QALY) gained. The projected incremental cost for pembrolizumab is S$141,979 compared to SoC, leading to an incremental cost-effectiveness ratio (ICER) of S$155,630 per QALY gained. A similar ICER of S$156,862 is observed in a scenario analysis whereby all patients are tested for PD-L1 and those with high PD-L1 expression are treated with pembrolizumab. With a Pembrolizumab Patient Access Program (PAP), the ICER is estimated to be S$95,279 per QALY. In another scenario analysis where government subsidies and claim limits of Medishield / Medisave are considered, the estimated ICER is S$46,308 per QALY from the Ministry of Health (MOH) perspective.
8eea62084ca7e541d918e823422bd82e Conclusion
For NSCLC patients with TPS≥50%, the ICER in the base-case for pembrolizumab as 1st-line treatment is $155,630 – which is between 2-3x gross domestic product (GDP) per capita of Singapore (S$73,167) in 2016, whereas the estimated ICER with Pembrolizumab PAP is 1-2x GDP per capita. Depending on threshold boundaries adopted, 1st line pembrolizumab for patients with similar profile (TPS ≥50%) as those in KN024, would be a cost-effective treatment compared to SoC in Singapore.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-109 - Real-World Patient-Reported Outcome Assessment of Patients with Metastatic Non-Small Cell Lung Cancer (ID 12213)
12:00 - 13:30 | Author(s): Sheenu Chandwani
- Abstract
Background
Patient-Reported Outcomes (PROs) provide information on patient treatment experience. We have established a real-world Advanced Non-Small Cell Lung Holistic Registry (ANCHoR) to understand how the advent of immunotherapy impacts treatment choice, clinical outcomes, and PROs of metastatic non-small cell lung cancer (mNSCLC). The aim of this analysis is to report early results of baseline symptom status and quality of life among mNSCLC patients using the MD Anderson Symptom Inventory lung cancer module (MDASI-LC) and EuroQol-5D 5-level version (EQ-5D-5L).
a9ded1e5ce5d75814730bb4caaf49419 Method
During 2017, patients with mNSCLC at a single institution were enrolled in ANCHoR and completed the PRO questionnaires at clinic visits. MDASI-LC consists of thirteen core and three lung cancer-specific symptom severity questions, and six interference items rated on 0-10 scales (0 = no symptom or interference, 10 = worst imaginable symptom or complete interference). EQ-5D-5L captures five health state dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression rated on a five-level scale (1= no problems, 5= extreme problems). A single visual analogue scale (VAS) on EQ-5D-5L records patient self-rated health between ”best imaginable” (100) and “worst imaginable” (0) health state. Descriptive statistics for PRO scores at baseline are summarized.
4c3880bb027f159e801041b1021e88e8 Result
Forty-two patients completed baseline PROs before the start of therapy. Mean patient age was 63 years and 45% were males. For MDASI-LC, the mean scores for the core symptom, lung cancer-specific symptom, and interference subscales at baseline were 2.2 (standard deviation [SD] = 2.80), 2.1 (SD = 2.80), and 2.8 (SD = 3.10), respectively. Fatigue was the most severe symptom reported at baseline (mean = 4.1, SD = 3.01), followed by shortness of breath (mean = 3.2, SD = 2.81) and pain (mean = 3.19, SD = 3.00). The highest percentages of patients reporting moderate to severe symptom levels (score of ≥5) were 38% for fatigue, 33% for pain, 31% for drowsiness, 29% for shortness of breath and disturbed sleep, and 26% coughing. For EQ-5D-5L, 91% of patient reported problems with self-care, 81% with mobility, 48% with usual activity and anxiety, and 33% with pain. Mean EQ-5D VAS was 73.9 (SD = 18.2).
8eea62084ca7e541d918e823422bd82e Conclusion
Prior to the start of treatment, fatigue, pain, drowsiness, disturbed sleep, and coughing were the most common symptoms with fatigue, shortness of breath, and pain being the most severe. Additional follow up will confirm and expand these findings and will also allow us to examine change in PROs after first-line treatment is administered.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P3.01-91 - Computing the Impact of Immunotherapy on the Non-Small Cell Lung Cancer (NSCLC) Therapeutic Landscape (ID 12209)
12:00 - 13:30 | Author(s): Sheenu Chandwani
- Abstract
Background
The Advanced Non-Small Lung Holistic Registry (ANCHoR) is established to examine the real-world impact of immunotherapy on choice of treatment, clinical outcomes, and patient reported outcomes of patients with Stage IV NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
Stage IV NSCLC patients diagnosed or initiating treatment at MD Anderson from January 1, 2017 are enrolled in the ongoing ANCHoR study. Their demographic, clinicopathological, molecular, and treatment data were populated in a prospective database. Treatment patterns by line and PD-L1 status were summarized in this interim analysis.
4c3880bb027f159e801041b1021e88e8 Result
At the time of data cut off (Dec 31, 2017) 182 patients were enrolled in the registry, of which 150 were tested for PD-L1. Number of patients initiating first-, second-, and third-line treatment were 163, 42 and 7, respectively. Of the 30 patients not tested for PD-L1, 10 did not have enough tissue and 8 had actionable mutations.
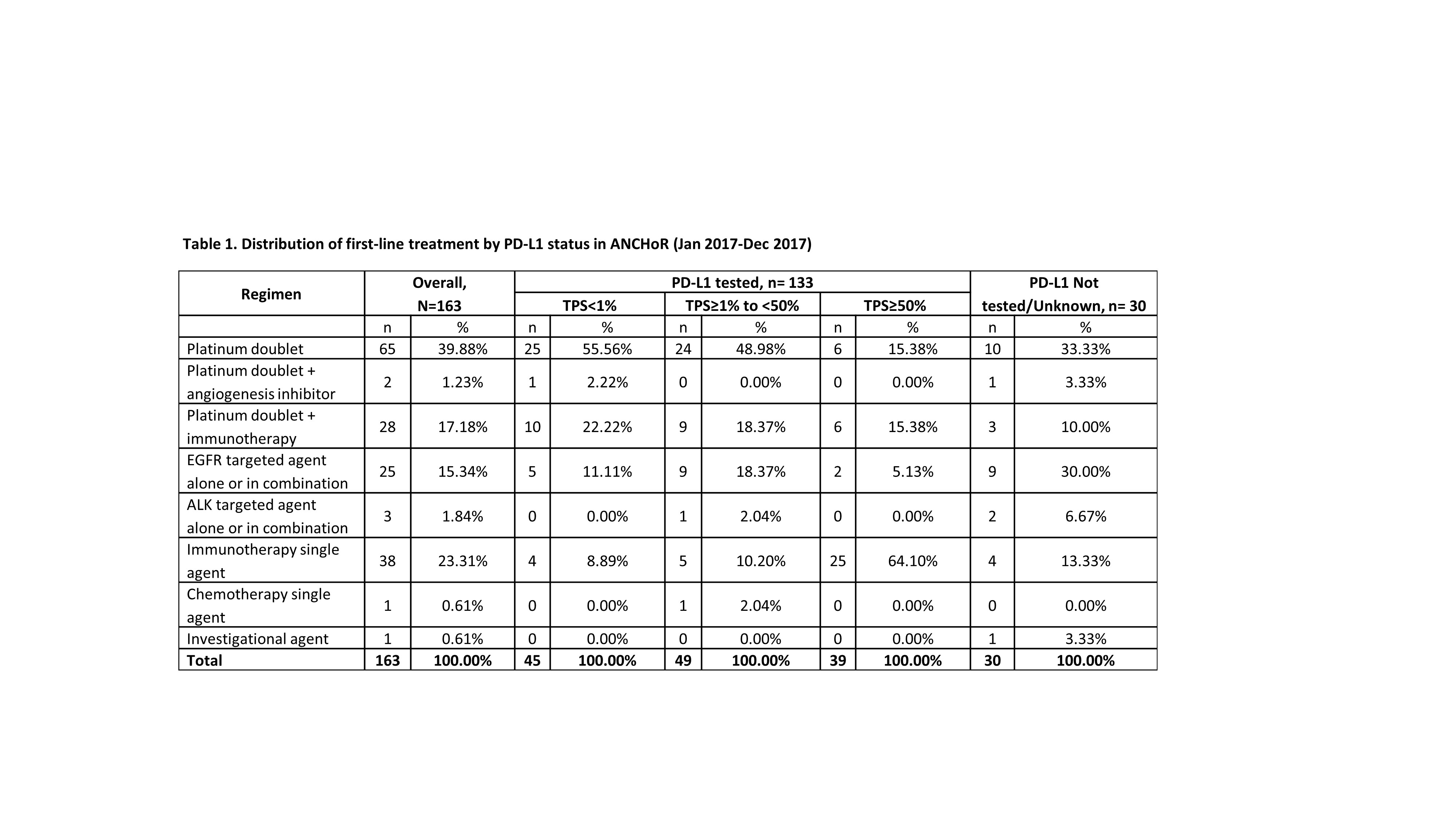
8eea62084ca7e541d918e823422bd82e Conclusion
The emergence of immunotherapy has had a dramatic impact on the first-line treatment of patient with advanced NSCLC. As of December, 2017 up to 41% of patient received immunotherapy either singly (23%) or in combination with chemotherapy. Only 40% of the patients now receive chemotherapy alone. There has been dramatic decrease in the use of chemotherapy with an anti-angiogenesis agent (1.23%). In our dataset 16% of the patients were eligible for targeted therapy as initial treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53


