Virtual Library
Start Your Search
John D Fenwick
Author of
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-31 - Safety and Tumour Hypoxia Modifying Effect of Buparlisib with Radiotherapy in NSCLC: A Phase I Dose Escalation Study (ID 12897)
16:45 - 18:00 | Author(s): John D Fenwick
- Abstract
Background
The Ras/PI3K/Akt pathway plays an important role in determining intrinsic and extrinsic tumour radiosensitivity. Buparlisib (BKM120) is a highly specific pan class-1 PI3K inhibitor. Pre-clinically, this class of agents radiosensitises tumours by direct effects on intrinsic radiosensitivity and by reducing tumour hypoxia. We therefore assessed the safety and determined the maximum tolerated dose (MTD) of buparlisib in combination with radiotherapy in patients with NSCLC and investigated its effect on tumour hypoxia.
a9ded1e5ce5d75814730bb4caaf49419 Method
BKM120 was a single centre, open-label, dose-escalation and dose-expansion phase 1 trial. Patients with advanced stage NSCLC received 2 weeks of oral buparlisib. Palliative thoracic radiotherapy (20Gy in 5 fractions) was delivered during the second week of treatment. 18F-fluoromisonidazole (FMISO) PET scans were used to assess tumour hypoxic volume (HV) before and after 1 week of buparlisib treatment. HV was defined as the volume with a tumour-to-blood F-MISO uptake ratio ≥ 1.4.
4c3880bb027f159e801041b1021e88e8 Result
From June 2013 to August 2017, 21 patients were recruited. 11 patients were registered to the dose escalation phase with 9 evaluable for MTD analysis: 3 in Cohort 1 (50mg OD), 3 in Cohort 2 (80mg OD) and 3 in Cohort 3 (100mg OD). No DLT was reported therefore 100mg OD was declared the MTD and 10 patients received this dose in the expansion phase. Of all patients who received buparlisib (n=21), 1 SAE (Grade 3 hypoalbuminaemia) was possibly related to buparlisib (5%). The most common buparlisib-related AEs were fatigue (8.3%) and nausea (3.3%). 93.9% of all AEs with any relation to buparlisib were ≤ Grade 2. There was no reported radiotherapy associated toxicity. 15 patients were evaluable for tumour hypoxia imaging analysis. Median change in HV in Cohort 1 (n=3), Cohort 2 (n=3) and Cohort 3 combined with the expansion cohort (n=9) was 7%, -18% and -20%, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
This is the first clinical trial of a specific PI3K inhibitor with concurrent radiotherapy in NSCLC. This combination was found to be safe and well-tolerated. This study provides clinical evidence that PI3K inhibition rapidly reduces tumour hypoxia and therefore warrants further trials combining this class of agents with radiotherapy.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-43 - ADSCaN: A Randomised Phase II Study of Accelerated, Dose Escalated, Sequential Chemo-Radiotherapy in Non-Small Cell Lung Cancer (NSCLC) (ID 13934)
16:45 - 18:00 | Author(s): John D Fenwick
- Abstract
Background
Lung cancer is the most common cause of cancer mortality in the UK, and NSCLC accounts for approximately 85% of all lung cancers. Most patients present with inoperable disease therefore radiotherapy plays a major role in treatment. However, the majority of patients are not suitable for gold standard treatment (concurrent chemo-radiotherapy) due to performance status and comorbidities. Novel strategies integrating radiotherapy advances and radiobiological knowledge need to be evaluated in patients treated with sequential chemo-radiotherapy. Four separate accelerated dose escalated radiotherapy schedules have been completed in UK (CHART-ED{1}, IDEAL-CRT{2}, I-START{3} and Isotoxic IMRT{4}). ADSCaN will compare these schedules with a UK standard sequential chemo-radiotherapy schedule. A combined randomized phase II screening / ‘pick the winner’ approach will identify the best schedule to take into a randomised phase III study against conventionally fractionated radiotherapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Suitable patients will have histologically / cytologically confirmed, stage III NSCLC and be able to undergo chemo-radiotherapy treatment. The study will recruit 360 patients; 130 on the standard arm and 60 on each experimental arm. Patients will complete 2-4 cycles of platinum based chemotherapy before being randomised to one of the radiotherapy schedules.
Logistic / capacity challenges make it impractical for sites to open all experimental trial arms; a novel trial design allows centres to select upfront the experimental arms they are able to participate in and all will offer the standard arm.
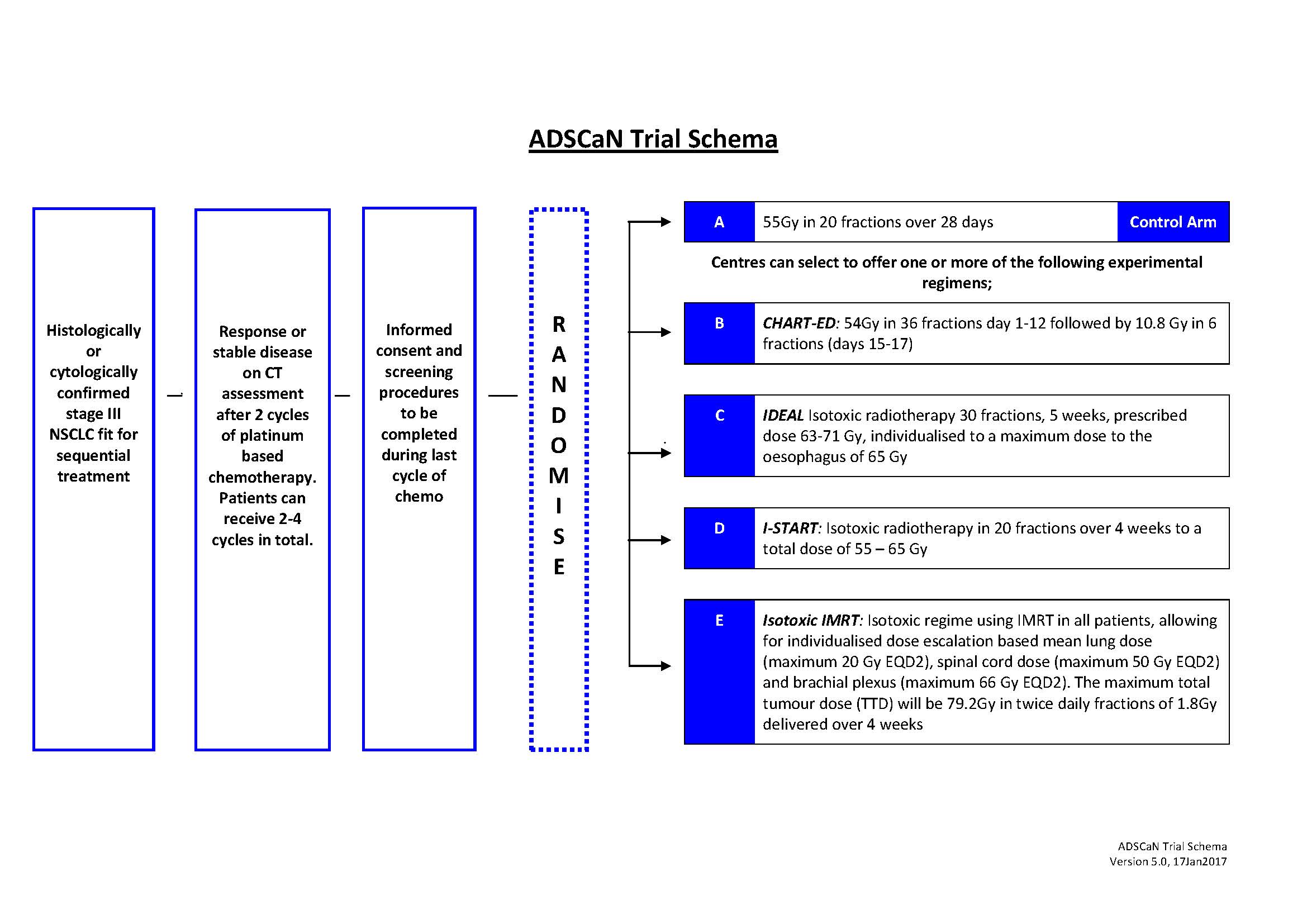
4c3880bb027f159e801041b1021e88e8 Result
CURRENT STATUS
CRUK is funding this multicentre study which is being co-ordinated by the CRUK CTU Glasgow. The study opened to recruitment on 22/08/2017 with planned recruitment lasting 3 years 8 months. The study includes a tailored QA programme through the UK RTTQA Group. 20 of the 36 sites expressing interest have started the QA process, 12 have completed with a further 8 expected to complete in the next few months.
8eea62084ca7e541d918e823422bd82e Conclusion
Section Not Applicable
6f8b794f3246b0c1e1780bb4d4d5dc53


