Virtual Library
Start Your Search
Michael Herman
Author of
-
+
P1.04 - Immunooncology (Not CME Accredited Session) (ID 936)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.04-23 - Expression of Emerging Immunotherapy Targets in Early-Stage Squamous Lung Carcinoma (ID 13520)
16:45 - 18:00 | Author(s): Michael Herman
- Abstract
Background
Anti-PD1/PD-L1 immunotherapy has demonstrated response in approximately 20% of unselected advanced non-small cell lung cancer (NSCLC) patients. Strategies involving combination immunotherapies are under investigation to improve the overall response to immunotherapy. The objective of this study was to identify the expression of emerging immune targets in a cohort of early-stage squamous lung carcinoma (SqLC), which may be used to design combinatorial immunotherapy approaches.
a9ded1e5ce5d75814730bb4caaf49419 Method
202 early stage (I-II) SqLC resected patient tumors and corresponding clinical data were collected from 6 cancer centers as part of the SPECS II program. Fourteen emerging immune targets or targeted axis were selected based on their advanced stage of development in preclinical/clinical studies. The mRNA expression level of these targets and PD-1/PD-L1 were determined by Affymetrix U133A gene expression profiling. The correlations among these targets and the overall survival were evaluated.
4c3880bb027f159e801041b1021e88e8 Result
The mRNA levels of the immune molecules which were grouped on PD-L1 protein expression in early stage SqLC are shown in Figure 1. No correlation was found between the mRNA level of PD-L1 and the other immune targets expressed on APC/tumor cells, except PD-L2 (r2= 0.41, p<0.00001). We found that the immune cell receptor, CD226, correlated with CD96 and CD112R respectively (r2= 0.514, p<0.00001; r2= 0.476, p<0.00001), and CD96 correlated with CD112R (r2= 0.644, p<0.00001) as well. In addition, higher expression of GAL-9, CD48 and ICOS were associated with better prognosis [p= 0.0358, HR=0.249 (0.068, 0.912); p= 0.0309, HR=1.61 (1.04, 2.49); p= 0.0429, HR=2.47 (1.03, 5.93)].
8eea62084ca7e541d918e823422bd82e Conclusion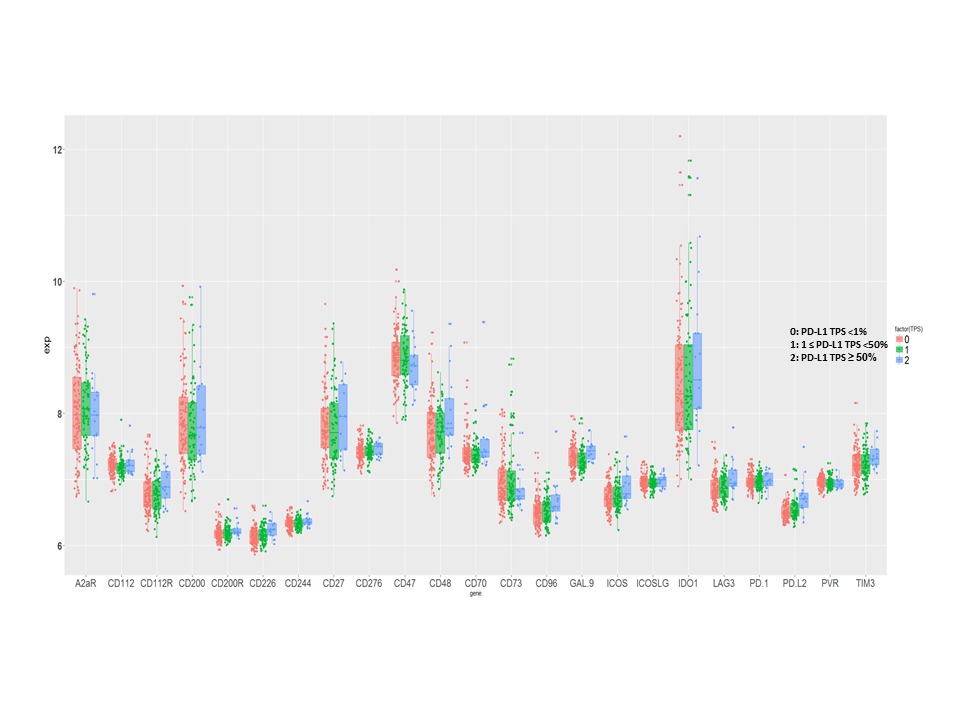
Several emerging immune targets were expressed at higher levels than PD-L1 in this early stage SqLC cohort. The mRNA levels of all immune targets evaluated were independent of PD-L1 expression, except PD-L2. The expression of GAL-9, CD48 and ICOS were identified as prognostic. These results may provide important information in the design of future combination immunotherapies for early-stage SqLC.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-46 - Investigating the Effects of Prior Malignancy on NSCLC Trial Eligibility (ID 12617)
16:45 - 18:00 | Presenting Author(s): Michael Herman
- Abstract
Background
Up to 18% of patients with non-small cell lung cancer (NSCLC) who are candidates for participation in a clinical trial are excluded due to a prior malignancy. Common reasons include the need for another anti-cancer therapy and potential impact on clinical trial endpoints. We evaluated the proportion of trials that exclude patients with a prior history of malignancy, and estimate the potential impact of the prior malignancy from a cohort of NSCLC patients treated with first line systemic therapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Clinical trials of palliative systemic therapy for NSCLC registered on or after January 1st 2014 from clinical trials.gov were reviewed to determine the proportion of trials that included a prior malignancy as an exclusion criterion. Patients with NSCLC and a prior malignancy, treated with first line palliative systemic treatment between January 1st 2010 and January 1st 2016 at the Princess Margaret Cancer Centre (PMCC), Toronto, Canada were identified from a cancer registry. A list of prespecified criteria of ways in which a prior malignancy may impact safety or a clinical trial endpoint was defined. These included: requirement for non-NSCLC anticancer therapies, other cancer detected on imaging, biopsy or tumour markers, disease progression or death from other malignancy, clinician uncertainty as to presence of synchronous other cancers. The proportions of patients with one or more of these criteria were assessed.
4c3880bb027f159e801041b1021e88e8 Result
66% of 359 clinical trial protocols contained an exclusion criterion for prior malignancy. 112 patients with advanced NSCLC and a prior malignancy were identified at PMCC, the median age was 71, 49% were male, the median time between prior cancer and NSCLC diagnosis was 74 months. 21% of patients were enrolled on a clinical trial. 52(59%) of the 88 patients not enrolled on a clinical trial did not meet any of our predetermined criteria, which may impact safety or a clinical trial endpoint due to other malignancy.
8eea62084ca7e541d918e823422bd82e Conclusion
A prior history of a malignancy is a common exclusion in NSCLC clinical trials. Preliminary results suggest the majority of patients in our study did not appear to have a complication that would have impacted safety or clinical trial endpoints. Further analyses to explore appropriate exclusion criteria for prior malignancies, which may minimize need to exclude patients from clinical trials, are underway.
6f8b794f3246b0c1e1780bb4d4d5dc53


