Virtual Library
Start Your Search
Elizabeth Dudnik
Author of
-
+
P1.04 - Immunooncology (Not CME Accredited Session) (ID 936)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.04-17 - Tumour Burden as a Predictive Tool of Response to Immune Checkpoint Inhibitors (ICI) in Patients with Metastatic Non-Small-Cell Lung Cancer (ID 12467)
16:45 - 18:00 | Author(s): Elizabeth Dudnik
- Abstract
Background
ICI are a novel class of agents that have revolutionized treatment for patients with metastatic non-small-cell lung cancer (NSCLC). Still, most patients do not benefit from PD-1 axis inhibitors, emphasizing the need for additional markers beyond PDL-1 expression for better selection of patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
This retrospective, single centre study included all consecutive patients with advanced NSCLC who were evaluated with a FDG-PET scan prior to first administration of an ICI (nivolumab or pembrolizumab) between 1/2016 and 6/2017. Tumour burden was calculated using the total body Metabolic Tumour Volume (MTV) and the sum of all measurable lesions (SOML) with accordance to the RECIST criteria. This study received IRB approval.
4c3880bb027f159e801041b1021e88e8 Result
A total of 58 patients with histologically proven NSCLC were included. Patients had a median age of 65 years (43-84), 59% were male, 62% had adenocarcinoma and 83% were previously treated with chemotherapy. The median PFS for the entire cohort was 5.7 (1-15.8) months, and the ORR for ICI was 44.8%.
The median MTV was 12.95 (0-236) millimeter³ and was significantly and inversely associated with longer PFS (p=0.036, 95%CI 1-1.015). The median SOML was 88 (13-305) centimetres, and was significantly and inversely associated with a longer PFS and higher ORR (PFS: P=0.004, 95% CI 1.002-1.011, ORR: OR 0.993 p=0.0067(.
Additionally, patients with a SOML under 56 CM (first quartile) had a longer PFS compared to patients with a higher disease volume (Table1).
SOML
(CM)
Median PFS
(months)P value
(compared to 1st quartile)1st quartile 56 12.1 - 2nd quartile 88 5.1 0.017 3rd quartile 115 4.23 0.036 4th quartile 305 3.15 0.01 table 1: some of measurable lesions (in centimeters) and PFS in metastatic NSCLC pateints recieving ICI
8eea62084ca7e541d918e823422bd82e Conclusion
In our study, a high tumour burden in patients with advanced NSCLC treated with ICI was associated with a shorter PFS and a lower ORR. This association warrants further prospective evaluation in order to optimize treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.04-30 - A Potential Effect of Diabetes Mellitus and Metformin Use on Efficacy of Immune Checkpoint Inhibitors (ICI) (ID 14072)
16:45 - 18:00 | Author(s): Elizabeth Dudnik
- Abstract
Background
Numerous studies have demonstrated metformin use is associated with decreased cancer risk in the general population, as well as improved overall response rate (ORR), progression free survival (PFS) and overall survival (OS) in cancer patients undergoing chemotherapy.Recent in-vitro studies found several new mechanisms which granted metformin the potential to increase cancer patients' response to immune checkpoint inhibitors (ICI).
a9ded1e5ce5d75814730bb4caaf49419 Method
In this study we aim to explore the correlation between the daily use of metformin and benefit from ICI in patients with lung cancer and other solid malignancies.
We retrospectively evaluated all consecutive patients with metastatic solid malignancies treated with ICI therapy in a single institution between February 2015 and June 2017. Patients' clinical data was obtained from electronic medical records. Cox proportional hazards model and chi squared test were used to determine the associations between metformin use and ORR, median PFS (mPFS) and median OS.
4c3880bb027f159e801041b1021e88e8 Result
Of 218 patients included in the analysis (202 NSCLC, 16 non lung cancers), 49 (22.5%) suffered from type 2 diabetes mellitus (T2DM). Of them 33 (15.1%) were treated with metformin and 16 (7.3%) received other, non-metformin therapy for T2DM. Comparison between non-diabetic and diabetic cancer patient groups demonstrated that mPFS was found to be significantly higher in the non-diabetic patients – 6.0 vs. 4.0 months (HR=1.47 [1.03-2.09], p=0.036). ORR was comparable (35.5% vs. 30.6%, p=0.52).
8eea62084ca7e541d918e823422bd82e Conclusion
In the T2DM subgroup - mPFS and HR suggested increased efficacy in the metformin group compared to non-metformin, but the numbers were too small to reach significance 8.0 vs. 3.2 months (HR=0.63 [0.32-1.23], p=0.17). ORR was also numerically higher (36.4% vs. 18.8%, p=0.21).
In both comparisons, no significant differences were found in OS.
This data suggests T2DM might be associated with decreased efficacy of ICI.
While several studies demonstrated that diabetic cancer patients receiving chemotherapy gained much benefit with metformin use, the trend we observed regarding metformin use with ICI therapy was milder and should be further explored in larger prospective cohorts.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-02 - Osimertinib for EGFR-Positive Advanced NSCLC with Brain Metastases: Preliminary Analysis of an Open-Label, Two-Arm, Phase 2 Study (ID 13020)
16:45 - 18:00 | Author(s): Elizabeth Dudnik
- Abstract
Background
Osimertinib is an EGFR tyrosine-kinase inhibitor (TKI) selective for both EGFR TKI sensitizing and Thr790Met resistance mutations. While intracranial activity of osimertinib was observed in EGFR-mutant NSCLC in larger trials, a study focusing on patients with brain metastases was not reported yet.
a9ded1e5ce5d75814730bb4caaf49419 Method
This phase 2, open-label, two-arm study enrolled patients with EGFR-mutant, advanced NSCLC and at least one asymptomatic brain metastasis. Treatment-naïve (arm A) and Thr790Met-positive patients who progressed on EGFR-TKI therapy (arm B) received osimertinib 80 mg QD. Dose escalation (160 mg QD) was performed in cases of intracranial progression without symptomatic systemic progression. The primary endpoint was intracranial metastasis response. The trial is ongoing (NCT02736513 at ClinicalTrials.gov) and here we present a preliminary analysis.
4c3880bb027f159e801041b1021e88e8 Result
Between May 31, 2016, and November 30, 2017 (data cutoff), 20 patients started osimertinib (arm A=15, arm B=5). Median duration of follow-up was 43 weeks. Intracranial response was achieved in 11 (73%; 95% CI 45%-92%) of 15 arm A and in four (80%; 95% CI 28%-99%) of five arm B patients. Dose escalation was performed in four cases (arm A=2, arm B=2), with one continuous response (25%, 95% CI 5%-70%). Ten of 15 patients (67%) in arm A and one of five patients (20%) in arm B continue responding to osimertinib 80 mg at data cutoff. Median intracranial PFS (80 mg) was not available for arm A (95% CI 232 days–NA), and was 510 days in arm B (95% CI 161–not available). Toxic effects were similar to previous reported data.
8eea62084ca7e541d918e823422bd82e Conclusion
Osimertinib shows equal intracranial and systemic activity with minor side-effects in EGFR-mutant NSCLC as first-line, as well as in previously treated Thr790Met-positive patients. It might therefore be a reasonable treatment for these patient populations and defer brain radiotherapy.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 961)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.12-14 - Stereotactic Radiosurgery for Brain Metastases in Small Cell Lung Cancer. (ID 13726)
16:45 - 18:00 | Presenting Author(s): Elizabeth Dudnik
- Abstract
Background
Prophylactic cranial irradiation (PCI) omission in favor of brain magnetic resonance imaging (MRI) staging and surveillance in the management of small cell lung cancer (SCLC) is controversial yet accepted by some centers policy. The latter strategy implies stereotactic radiosurgery (SRS) treatment (Tx) for limited brain metastases (BM). Data regarding SRS efficacy in this setting is limited.
a9ded1e5ce5d75814730bb4caaf49419 Method
Ten consecutive SCLC patients (pts) with BM treated with SRS at Davidoff Cancer center between Aug 2012 and July 2017 were identified through the institutional database; pts receiving PCI or whole brain radiotherapy (WBRT) as a primary intervention were excluded. Brain MRI images were reviewed by neuro-radiology specialist. Intracranial objective response rate (IORR), progression-free survival (IPFS), intracranial failure patterns, overall survival (OS) and time-to-WBRT/death whichever occurred first (TTWD) were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
Baseline pt characteristics are presented in the Table. SRS dose ranged from 16 Gy to 22.5 Gy. IORR comprised 57% by RECIST 1.1 and 60% by mRECIST 1.1. Intracranial progression developed in 8 pts; median IPFS was 3.9 mo (95% CI, 1.7-7.2). In-site, off-site and combined pattern of intracranial failure was seen in 0, 6, and 2 pts, respectively; median number of new BM per disease course was 2 (range, 1-11). Nine additional rounds of SRS were delivered in 6 pts (median number of lesion irradiated per round-1, range, 1-5). WBRT was ultimately administered in 3 pts; 4 pts died. Median TTWD comprised 23.2 mo (95% CI, 1.9-26.8). Median OS since SRS administration was 23.2 mo (95% CI, 4.2-26.8).
8eea62084ca7e541d918e823422bd82e Conclusion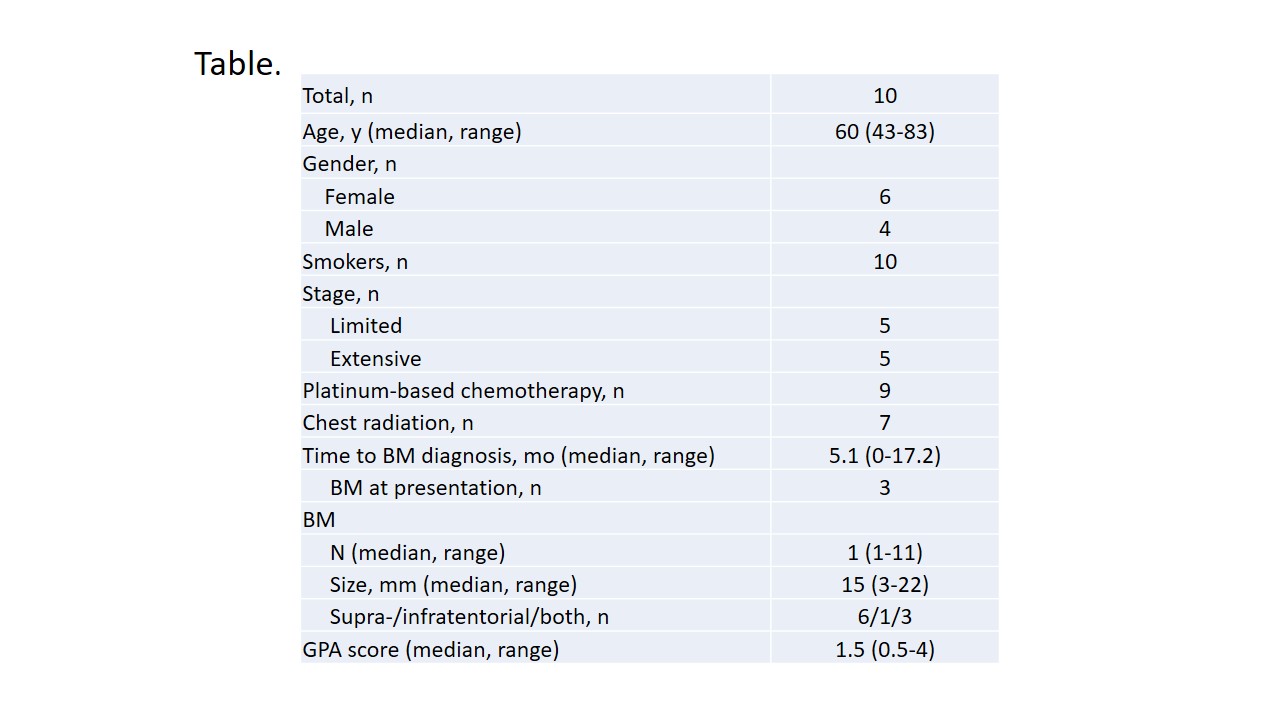
MRI surveillance combined with multiple rounds of SRS in case of limited BM development might provide reasonable alternative to PCI or therapeutic WBRT in SCLC. SRS in SCLC warrants prospective evaluation.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-28 - The Clinical Impact of Comprehensive cfDNA Genomic Testing in Lung Cancer (ID 13878)
12:00 - 13:30 | Author(s): Elizabeth Dudnik
- Abstract
Background
Next-generation sequencing (NGS) of cell-free circulating tumor DNA (cfDNA) enables a non-invasive option for comprehensive genomic analysis of non-small cell lung cancer (NSCLC) patients. Although plasma-detected genomic alterations have been shown to predict targeted therapy response, evidence of durability of response is lacking or limited to small cohorts as is the impact of cfDNA NGS results on clinical decision making.
a9ded1e5ce5d75814730bb4caaf49419 Method
In this retrospective study, data was collected on stage IIIB/IV NSCLC patients between the years 2014-2017 in Israel. We utilized cfDNA NGS (Guardant360) which covers the seven genes targetable with FDA-approved therapies in NSCLC.
4c3880bb027f159e801041b1021e88e8 Result
116 consecutively NSCLC patients were tested, 41.4% (48/116) before 1st line therapy (Group A), 34.5% (40/116) upon progression on chemotherapy or immunotherapy (Group B1) and 24.1% (28/116) upon progression on EGFR TKIs (Group B2). Targetable genomic alterations were found in 65% of group A (15/48), 53% in group B1 (21/40) and 71% in group B2 (20/28). Treatment decision was changed to targeted therapy based on cfDNA NGS analysis in 23% (11/48), 25% (10/40) and 32% (9/28), respectively (total cohort 26%; 30/116). Response assessment (RECIST) showed complete response in 4% (1/28), partial response in 39% (11/28), stable disease in 32% (9/28) and progressive disease in 25% (7/28). Total objective response rate (ORR) was 43% and disease control rate was 75% for 5 months treatment duration.
8eea62084ca7e541d918e823422bd82e Conclusion
Comprehensive cfDNA testing impacted clinical decisions in 23% of naïve patients, 25% in patients who progressed on chemotherapy and 32% in EGFR TKI progressors. Median treatment duration was 5 months. This retrospective study extends previous reports by showing that responses based on cfDNA are durable and change treatment decisions at initial presentation and at progression.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.04 - Immunooncology (Not CME Accredited Session) (ID 970)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.04-28 - Can Ipilimumab Restore Immune Response in Advanced NSCLC After Progression on Anti PD1/PDL1 Agents? (ID 13775)
12:00 - 13:30 | Author(s): Elizabeth Dudnik
- Abstract
Background
Anti PD1/PDL1 agents play a crucial part in the treatment of NSCLC demonstrating improved overall response rate (ORR) and overall survival. While anti CTLA4 alone did not show significant single agent activity, a phase 1 study evaluating the combination with anti PD1 suggests improved ORR.
Evidence is scarce regarding subsequent treatment with immune checkpoint inhibitors (ICI) after progression on anti PD1/PDL1. A recent study in melanoma patients reported lack of benefit from the addition of Ipilimumab after progression on anti PD1.
a9ded1e5ce5d75814730bb4caaf49419 Method
All off-label Ipilimumab administrations in a single tertiary center were retrieved through the institutional review board records. 21 patients with advanced NSCLC were allocated during 2017. Clinical data were retrieved retrospectively. Disease control was define as partial response (PR) or stable disease (SD).
4c3880bb027f159e801041b1021e88e8 Result
Table 1 - Patients’ characteristics N-15 (%) Sex M-15, F-0 Median age (range) 67 (53-87)
Histology Adenocarcinoma
12 (80%)
Squamous
1 (6.7%)
Adenosquamous 1 (6.7%)
NSCLC-NOS 1 (6.7%) PD-L1 expression >50%
2 (13.3%)
1-50%
4 (26.7%)
<1%
2 (13.3%)
Unknown 7 (46.7%) Line of Tx with anti PD1/PDL1 1st
2 (13.3%)
2nd 12 (80%)
3rd 1 (6.7%) Best response to anti PD1/PDL1 PR 5 (33.3%)
SD
2 (13.3%)
PD 8 (53.4%) Median time to progression on anti PD1 (range) 9 mon. (6-17) Of 21 patients who were treated with a combination of anti PD1 agent and Ipilimumab, in 15 Ipilimumab was initiated after confirmed progression on anti PD1/PDL1 alone. Patients' characteristics are described in table 1.
The overall disease control rate was 33.3% (n-5); 3 patients with PR and 2 patients with SD, 3 of them had previously responded to anti PD1. Immune related AE rate was 40% (n-6); 2 patients had grade 3 AE and one patient died of pneumonitis. While the median time to progression was 2 months, 4 of the 5 responding patients are still stable after median 4 months follow up (range 1-15).
8eea62084ca7e541d918e823422bd82e Conclusion
This small retrospective cohort suggests Ipilimumab might re-boost immune response in patients with advanced NSCLC progressing on anti PD1 therapy, while delaying exposure to the higher rates of AE associated with upfront combination therapy. This strategy should be explored prospectively.
6f8b794f3246b0c1e1780bb4d4d5dc53


