Virtual Library
Start Your Search
Goulnar Kasymjanova
Author of
-
+
P1.04 - Immunooncology (Not CME Accredited Session) (ID 936)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.04-16 - Comparison of Clinical Response to Checkpoint Inhibitors in Advanced NSCLC with High PD-L1 Expression Tested on Cytology Versus Biopsy Samples (ID 12525)
16:45 - 18:00 | Author(s): Goulnar Kasymjanova
- Abstract
Background
PD-L1 immunohistochemistry (IHC) expression correlates with clinical response to checkpoint inhibitors in advanced-stage NSCLC. PD-L1 IHC testing is usually performed on tissue blocks from core needle biopsy or surgical resection, but appears to be feasible on cytology cell blocks as well. In this retrospective study, we assessed the clinical response to checkpoint inhibitors in patients with NSCLC and high PDL1 IHC expression on cytology specimens in comparison with tissue biopsy specimens.
a9ded1e5ce5d75814730bb4caaf49419 Method
Between August 2015 and April 2018, 116 patients with NSCLC received immunotherapy at our institution. Only cases with known PD-L1 expression from IHC testing performed on small biopsies or cytology cell blocks were included. A total of 65 consecutive cases were reviewed, including 40 small biopsies and 25 cytology samples. A Tumor Proportion Score (TPS) was categorized as high (≥ 50% tumor cell staining) or low (<50%). Response to treatment was categorized as disease control (DC, including complete and partial response and stable disease) or progression (PD). The primary outcome was the rate of disease control.
4c3880bb027f159e801041b1021e88e8 Result
Patients were mostly current or ex-smokers (91%), Caucasians (82%) and non-squamous carcinomas (85 %). High TPS was seen in 44 (68%) cases. Immunotherapy was given in the first line setting in 20 (31%) patients, the second line in 36 (55%), and the third line in 9 (14%). 50 (77%) patients received Pembrolizumab, 10 (15%) Nivolumab and 5 (8%) others received immunotherapies on RCTs. Overall, there was DC in 40 (62%) patients and PD in 17 (26%). There was no significant difference in DC rate between the cytology and the small biopsy groups in high TPS group.
8eea62084ca7e541d918e823422bd82e Conclusion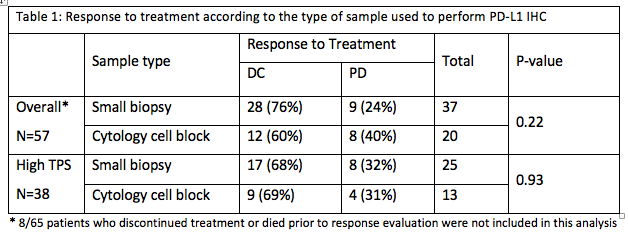
PD-L1 expression on cytology cell blocks and on small biopsies appears to have similar clinical significance. Further prospective trials are needed to confirm these findings.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.09 - Pathology (Not CME Accredited Session) (ID 958)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.09-07 - Does Metastatic Site Matter for PD-L1 Testing in Stage IV NSCLC? (ID 13385)
16:45 - 18:00 | Author(s): Goulnar Kasymjanova
- Abstract
Background
Stage IV non-small cell lung cancer (NSCLC) often presents with metastasis to multiple distant sites. Currently PD-L1 expression by immunohistochemistry (IHC) testing with Tumor Proportion Score (TPS) ≥ 50% and ≥1% is required for first- and second-line Pembrolizumab treatment respectively. However, it is not well known if PD-L1 expression differs in NSCLC specimens sampled from different distant metastatic sites. In this study, we evaluate PD-L1 expression in distant metastatic sites.
a9ded1e5ce5d75814730bb4caaf49419 Method
A total of 400 NSCLC specimens from distant metastatic sites are included in this study. The metastatic sites include brain, bone, non-regional lymph nodes, serous membranes (pleura, pericardium and peritoneum) and organs outside the chest (liver, adrenal gland, skin, soft tissue). The samples are either cytology cell blocks, small biopsies or surgical resections. IHC was performed using Dako PD-L1 IHC 22C3 pharmDx. A total of 100 viable tumor cells is required for adequacy. TPS≥ 50% and 1-49% are defined as high and low PD-L1 expression respectively.
4c3880bb027f159e801041b1021e88e8 Result
Overall, the rate of TPS >50% ranges from 36-47% in different metastatic organ sites (Table 1). The prevalence of PD-L1 high and low expression is similar for all distant metastatic sites (P=0.91). Brain metastases have a slightly lower rate of high PD-L1 expression but the difference is not statistically significant.
Table 1. PD-L1 expression in different metastatic sites
Metastatic sites
Tumor Proportion Score (TPS)
Total
≥50%
n (%)
1-49%
n (%)
0%
n (%)
n (%)
Brain
13(36%)
9 (25%)
14(39%)
36 (100%)
Bone
21(44%)
11(23%)
16(33%)
48 (100%)
Nonregional lymph nodes
6(40%)
3(20%)
6 (40%)
15 (100%)
Serous membranes
91(40%)
62(27%)
74(33%)
227 (100%)
Organ outside chest
35(47%)
20(27%)
19(26%)
74 (100%)
Total
166(42%)
105(26%)
129(32%)
400 (100%)
P=0.91
8eea62084ca7e541d918e823422bd82e Conclusion
Our results suggest that the specimens for PD-L1 IHC testing can be sampled from any accessible distant metastatic site.
6f8b794f3246b0c1e1780bb4d4d5dc53


