Virtual Library
Start Your Search
Jun Nakajima
Author of
-
+
MA19 - Genomic Markers of IO Response (ID 922)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Immunooncology
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 15:15 - 16:45, Room 201 BD
-
+
MA19.05 - Difference of Tumor Mutational Burden Is Associated with Distinct Immune Microenviroment in the T Cell-Inflamed Lung Adenocarcinoma (ID 13495)
15:40 - 15:45 | Author(s): Jun Nakajima
- Abstract
- Presentation
Background
PD-L1 expression on tumor cells, tumor infiltrating lymphocytes (TILs), and tumor mutational burden (TMB) have been reported as predictive biomarkers for checkpoint inhibitor immunotherapies. However, little is known about the relationship between each biomarkers. The aim of this study was to assess the relationship between these biomarkers, especially TIL and TMB.
a9ded1e5ce5d75814730bb4caaf49419 Method
RNA-seq data of 533 primary lung adenocarcinoma were downloaded from The Cancer Genome Atlas (TCGA). Gene expression and gene set enrichment were analyzed. Clinical information and somatic missense mutation data were also integrated.
4c3880bb027f159e801041b1021e88e8 Result
Weak correlation between PD-L1 and CD8A expression (Spearman’s R=0.32, P<0.001), and PD-L1 expression and TMB (R=0.10, P=0.019) were seen, but not between CD8A expression and TMB (R=0.03, P=0.45). Next, we performed gene signature analysis related to cancer-immunity cycle (ref. Karasaki et al. J Thorac Oncol 2017). Hierarchical clustering resulted in 3 clusters: T cell non-inflamed phenotype with high antigenicity (Cluster 1), inflamed phenotype with low antigenicity (Cluster 2), and inflamed phenotype with high antigenicity (Cluster 3).(Fig.1) Compared with Cluster 3, Cluster 2 was featured by lower gene expression signature of cytolytic activity (P<0.0001, U-test), as well as lower expression of PD-L1 (P<0.0001, U-test).
To further investigate the relationship between TMB and TILs, T-cell inflamed phenotype tumors were divided into four groups according to the quartiles of TMB. We estimated immune cell phenotypes of TMB-high (upper quartile) and TMB-low (lower quartile) groups using ssGSEA and CIBERSORT. Either analysis showed significant enrichment of activated CD4 T cells in TMB-high group (P<0.0001, T-test).
8eea62084ca7e541d918e823422bd82e Conclusion
Existence of Cluster 1 and 2 suggested that tumor antigenicity (TMB) does not necessarily correlate with TIL enrichment. TMB-low tumors may form T cell-inflamed tumors (Cluster 2), although the immune status may differ from TMB-high inflamed tumors (Cluster 3). Integrating multiple biomarkers for the assessment of tumor immune microenvironment is important for optimal immunotherapy.
6f8b794f3246b0c1e1780bb4d4d5dc53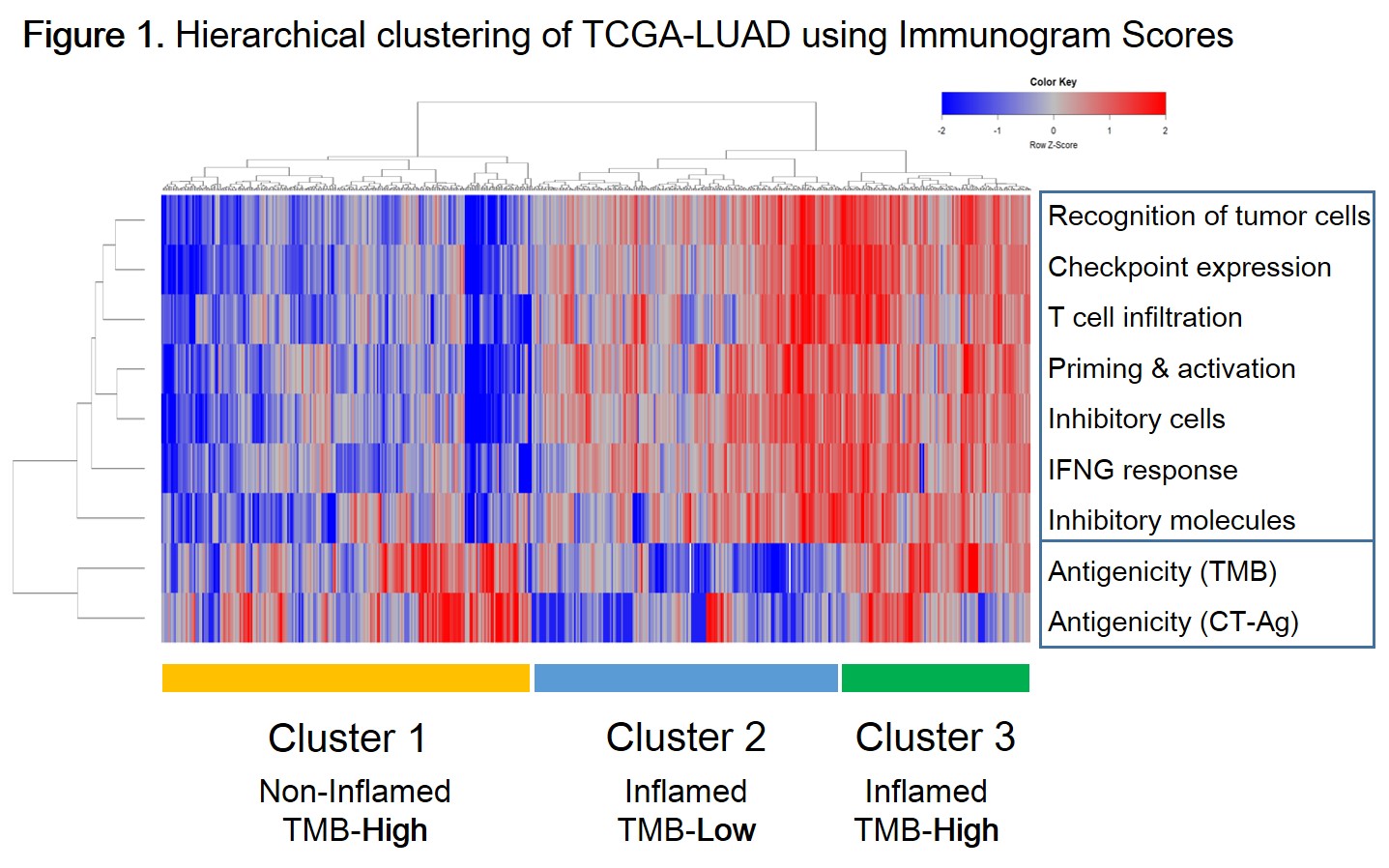
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 948)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.16-45 - Thoracoscopic Stapler-Based Complex Segmentectomy Assisted by Virtual Assisted Lung Mapping (ID 14369)
16:45 - 18:00 | Author(s): Jun Nakajima
- Abstract
Background
Anatomical segmentectomies play an important role for the patients with small ground-glass lung cancer and metastatic lung tumors. Virtual assisted lung mapping (VAL-MAP) assists to localize hardly palpable lung tumors and to define the resection lines. VAL-MAPhas been found to be useful in thoracoscopic segmentectomies, particularly complex segmentectomies.
VAL-MAP is a novel preoperative bronchoscopic multi-spot dye-marking technique to provide “geometric information” to the lung surface, using three-dimensional virtual images.The multiple spots (tattooing) of VAL-MAP assists not only to localize hardly palpable lung tumors but to define appropriate resection lines in sublobar lung resections including wedge resections and segmentectomies. The purpose of the study is to evaluate the role of VAL-MAP in simple and complex thoracoscopic segmentectomy.
a9ded1e5ce5d75814730bb4caaf49419 Method
VAL-MAP was conducted before surgery as follows: the target bronchi were identified using radiology workstation; 1 ml of indigo carmine was injected through a catheter by bronchoscopy under fluoroscopy; another CT scan was taken to confirm marking locations. Segmentectomies were conducted thoracoscopically and intersegmental planes were made using staplers (Figure). Anatomical segmentectomies using VAL-MAP conducted were retrospectively analyzed (2014-2017). Simple segmentectomy was defined as a resection of anatomical single segment or combined segments. Other anatomical segmentectomies were defined as complex segmentectomy (Table). Successful resection rates, surgical margins, and post-operative course were compared between simple segmentectomy and complex segmentectomy.
4c3880bb027f159e801041b1021e88e8 Result
Atotal of 43 patients were included in the study (42% women; median age 68 yr (48-83 yrs.). The median tumor size was 12 mm. The average number of markings via VAL-MAP was 4.2 (1-8) points. Post-VAL-MAP complications identified in CT included 5 pneumothorax, 3 airway bleeding, and 2 mediastinal emphysema, although none needed additional treatment.Simple segmentectomy was conducted in 18 patients, while complex segmentectomy was conducted in 25 patients. There was no significant difference in operation time (simple vs. complex; 233±100 vs. 266±8 min), length of chest tube drainage (2.3±0.33 vs. 2.5±0.25 days), or margin/tumor diameter ratio (2.10±0.28 vs. 1.89±0.23). Minor postoperative complicationswere found in 4 patients (2 collapse lung after removed chest tube; 2 pneumonia and inflammatory reaction). No pleurodesis was needed postoperatively.Final pathology included 24 lung cancer, 15 metastatic tumors, and 3 others.
8eea62084ca7e541d918e823422bd82e Conclusion
VAL-MAP-assisted stapler-based thoracoscopic segmentectomies were safely and effectively conducted even in complex ones. The bronchoscopic approach of VAL-MAP made the anatomical liberty available to surgeons. The “map” drawn with VAL-MAP not only helps to identify the tumor, but also helps to determine oncologically appropriate resection lines.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 965)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.16-40 - Impact of Preoperative Pectoralis Muscle Quantity and Density on Outcome After Complete Resection of Non-Small Cell Lung Cancer (ID 12135)
16:45 - 18:00 | Author(s): Jun Nakajima
- Abstract
Background
Body composition measures may predict outcomes of cancer surgery. In this study we evaluated the prognostic significance of pectoralis muscle quantity and density in patients with surgical non-small cell lung cancer.
a9ded1e5ce5d75814730bb4caaf49419 Method
Preoperative pectoralis muscle quantity and density were retrospectively assessed in 181 patients undergoing lobectomy and lymph node dissection for non-small cell lung cancer from 2009 to 2013. The pectoralis muscle index (cross-sectional area/height2) and density (average Hounsfield unit, HU) at the fourth thoracic vertebra level were measured and calculated on preoperative plain computed tomography. Overall survival was analyzed between the lowest gender-specific quartile of the pectoralis muscle index and density and the other quartiles.
4c3880bb027f159e801041b1021e88e8 Result
Positive correlations between pectoralis muscle index and density and body mass index (BMI) were identified in the cohort (Pearson's r=0.349, p<0.001; r=0.206, p=0.005, respectively). The gender-specific lowest quartile cut-off values of the pectoralis muscle index and density was 10.14cm2/m2 and 28.97HU for males, 7.86cm2/m2 and 21.23HU for females, respectively. The cumulative five-year overall survival rates were significantly shorter in patients with low pectoralis muscle index (51.7% vs. 76.0%, p=0.009), while for low pectoralis density (66.0% vs. 70.7%, p=0.391). The multivariate analysis including age, smoke index, BMI, c-reactive protein, carcinoembryonic antigen and pathologic stage revealed that the pectoralis muscle index, not the pectoralis density or BMI, was an adverse independent risk factor for overall survival (p=0.002, hazard ratio: 2.815, 95% confidence interval: 1.473–5.377).

8eea62084ca7e541d918e823422bd82e Conclusion
A low preoperative pectoralis muscle index was associated with a poor postoperative outcome in surgical patients with non-small cell lung cancer. Pectoralis muscle quantity which is more predictive than density and BMI as a convenient measure, may be included in the preoperative assessment when surgical intervention is considered for non-small cell lung cancer.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.08 - Oligometastatic NSCLC (Not CME Accredited Session) (ID 974)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.08-08 - Outcomes of Pulmonary Metastasectomy in Breast Cancer: Prognosis Based on the Metastatic Lung Tumor Study Group of Japan (ID 12242)
12:00 - 13:30 | Author(s): Jun Nakajima
- Abstract
Background
Although pulmonary metastasectomy is a common treatment in other primary cancers, its role in patients with primary breast cancer is still controversial. The purpose of this study was to analyze a Japanese multi-center database to assess the prognostic factors and indications of metastasectomy in breast cancer.
a9ded1e5ce5d75814730bb4caaf49419 Method
Data of 387 patients with histologically proven pulmonary metastases from breast cancer have been prospectively enrolled in the Metastatic Lung Tumor Study Group of Japan between December 1982 and March 2017. Those with inadequate information about perioperative data, surgery for biopsy, male or non–epithelial malignancies were excluded. A total of 253 female with invasive breast carcinoma between 1982 and 2017 constituted the study population, and their clinical and prognostic data were retrospectively analyzed.
4c3880bb027f159e801041b1021e88e8 Result
The median follow-up period was 5.4 (range, 0–24) years. The mean age of patients was 56 (range, 32–82) years, the median disease-free interval was 4.8 (range, 0–31) years, pulmonary metastasis (215 solitary, 38 multiple) was treated with surgeries, namely, wedge resection (n = 113, 45%), segmentectomy (n = 22, 9%), lobectomy (n = 116, 45%), and pneumonectomy (n = 2, 1%). Nodal metastases were found in 56 (22%) patients. There were 24 (9%) patients with incomplete resection. Additional treatments after metastasectomy were performed in 141 patients (56%). Recurrence after pulmonary metastasectomy developed in 98 of 229 (43%) patients without incomplete resection, namely, intrathoracic lesion (n = 21, 23%), distant metastasis (n = 47, 51%), and unknown (n = 25, 27%). The 5-year and 10-year survival rates after pulmonary metastasectomy were 66% and 52%, respectively, and the median survival period was 10 years. In the univariate analysis, early treatment period (<2001; p = 0.02), short disease-free interval (<3 years; p < 0.01), large tumor size (>2 cm; p < 0.01), surgical procedure (lobectomy and pneumonectomy; p = 0.01), intrathoracic nodal metastasis (p < 0.01), and incomplete resection (p = 0.05) were shown to be associated with poor survival. Multivariate analysis revealed that only short disease-free interval (<3 years; p < 0.01) was significantly worse prognostic factor in 253 patients.
8eea62084ca7e541d918e823422bd82e Conclusion
The main poor prognostic factor was disease-free interval (<3 years). However, complete resection of metastases was not a significant prognostic factor. The efficacy of pulmonary metastasectomy in breast cancer is still controversy. For pulmonary metastais from breast cancer, pulmonary metastasectomy is considered to be optional treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 982)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.16-43 - Is Sublobar Resection for Stage I Invasive Adenocarcinoma (≤2-cm) Feasible? (ID 13545)
12:00 - 13:30 | Author(s): Jun Nakajima
- Abstract
Background
Recent studies have reported that sublobar resection is not inferior to lobectomy for small-sized non-invasive adenocarcinoma (ADC); however, the adequacy for small-sized invasive ADC (IAD) remains unclear. The objective of this study was to identify prognostic factors and validate sublobar resection for small-sized IAD.
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively reviewed patients with therapy-naïve, pathological stage I (≤2-cm) IAD, who had undergone complete resection from 1998-2015. Each tumor was evaluated by comprehensive histologic subtyping according to the 2015 World Health Organization classification. Overall survival (OS) and recurrence-free survival (RFS) was estimated using the Kaplan-Meier method.
4c3880bb027f159e801041b1021e88e8 Result
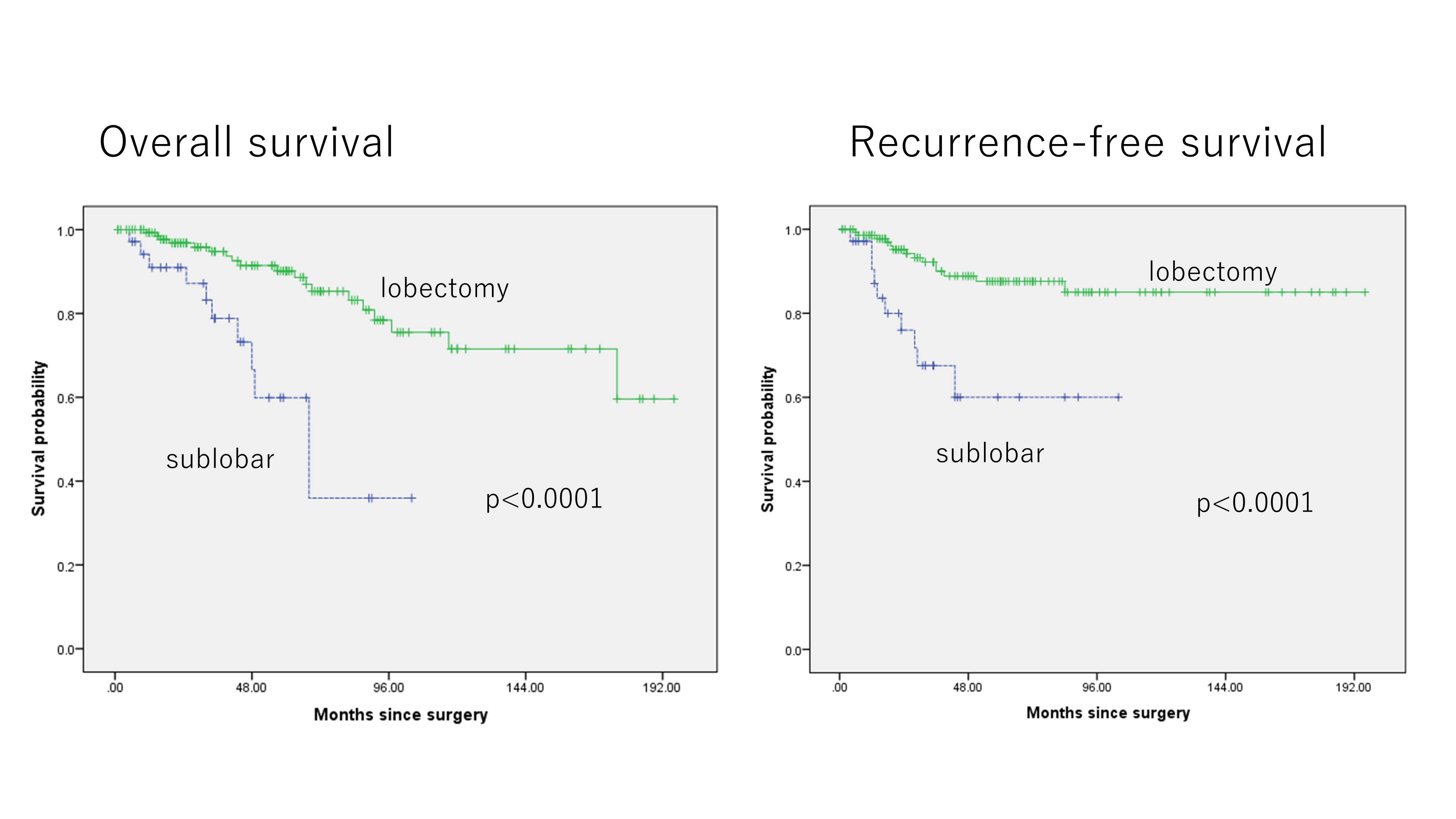 179 patients met inclusion criteria. 104 (58%) were male and 75 (42%) were female, with a median age of 68 years; sublobar resection was performed in 35 (20%), and lobectomy or pneumonectomy in 144 (80%). Median pathological tumor size was 1.5 cm, with a median invasive component size of 0.9 cm, and pleural, lymphatic, or vascular invasion in 27 (15%), 25 (14%), and 49 (23%) patients, respectively. In IAD, the elderly patients and ever smokers were likely to undergo sublobar resection (p=0.015, 0.011, respectively). Patients undergoing sublobar resection for IAD had significantly worse prognosis (5-year OS: 59.9%, 90.2%, p<0.0001) and increased risk of local recurrence (5-year RFS: 60.0%, 87.6%, p<0.0001). Multivariable analysis revealed that sublobar resection, age, and sex was an independent risk factor of overall survival and sublobar resection and vascular invasion was an independent risk factor of recurrence for IAD ≤2-cm.
179 patients met inclusion criteria. 104 (58%) were male and 75 (42%) were female, with a median age of 68 years; sublobar resection was performed in 35 (20%), and lobectomy or pneumonectomy in 144 (80%). Median pathological tumor size was 1.5 cm, with a median invasive component size of 0.9 cm, and pleural, lymphatic, or vascular invasion in 27 (15%), 25 (14%), and 49 (23%) patients, respectively. In IAD, the elderly patients and ever smokers were likely to undergo sublobar resection (p=0.015, 0.011, respectively). Patients undergoing sublobar resection for IAD had significantly worse prognosis (5-year OS: 59.9%, 90.2%, p<0.0001) and increased risk of local recurrence (5-year RFS: 60.0%, 87.6%, p<0.0001). Multivariable analysis revealed that sublobar resection, age, and sex was an independent risk factor of overall survival and sublobar resection and vascular invasion was an independent risk factor of recurrence for IAD ≤2-cm.
8eea62084ca7e541d918e823422bd82e Conclusion
Sublobar resection in patients with IAD ≤2-cm was significantly associated with increased risk of recurrence and worse prognosis.
6f8b794f3246b0c1e1780bb4d4d5dc53


