Virtual Library
Start Your Search
Ryan N. Walton
Author of
-
+
MA18 - Modelling, Decision-Making and Population-Based Outcomes (ID 920)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment in the Real World - Support, Survivorship, Systems Research
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 201 F
-
+
MA18.09 - Predictors of Health Utility Scores (HUS) in Advanced EGFR-Mutated NSCLC. (ID 13087)
14:25 - 14:30 | Author(s): Ryan N. Walton
- Abstract
- Presentation
Background
Advanced NSCLC patients with EGFR mutations (EGFRm) are currently treated with first - to third-generation tyrosine kinase inhibitors (TKIs). In the advanced setting, quality of life is an important goal; we therefore evaluated determinants of HUS in this population.
a9ded1e5ce5d75814730bb4caaf49419 Method
In a prospective, observational study, patients with advanced EGFRm NSCLC completed EQ-5D surveys at outpatient visits generating HUS (range 0-1). Patients were allowed to enrol at any point in their disease course. Baseline clinical characteristics and outcome data were extracted from chart review. Patient imaging was reviewed and health states (stable/progressing) at each encounter recorded. Univariable analyses conducted using ANOVA and multivariable regression analyses with generalized estimating equations identified factors associated with HUS.
4c3880bb027f159e801041b1021e88e8 Result
From November 2014 to July 2017, 782 encounters (follow-up visits) were collected for 244 patients. Median age at first encounter was 64 years (range:29-96); 54% were female and 54% Asian. Median time from diagnosis of stage IV NSCLC to first encounter was 23 months (range:0-67). The median number of HUS collected per patient was 2 (range:1-14). For patients with multiple visits the median time between completed questionnaires was 1.8 months (1-18). 105 patients (43%) presented with or developed brain metastases during the study period. In a univariable analysis, regardless of treatment line, mean HUS (mHUS) on osimertinib was 0.85 (standard deviation (SD):0.15) (n=33 patients; 114 encounters) compared to mHUS=0.80 (SD:0.17) on gefitinib (n=147, 351 encounters); mHUS=0.72 (SD:0.16) on chemotherapy (n=32, 76 encounters); and mHUS=0.79 (SD=0.15) on other TKIs (n=49, 133 encounters); p<0.001. In a multivariable analysis, disease progression (p=0.04) and ECOG performance status >0 (p<0.001) were associated with lower HUS. In contrast, treatment with osimertinib (when compared to a reference group of first-generation TKIs, gefitinib/erlotinib) was associated with improved HUS (p=0.01), while line of therapy and number of metastatic sites of disease were not associated with HUS. In addition, brain metastases had no significant impact on HUS (p=0.33).
8eea62084ca7e541d918e823422bd82e Conclusion
Progressive disease and worse performance status associate with lower HUS in patients with EGFRm NSCLC. Patients treated with osimertinib had the highest HUS when compared with a reference group of first-generation EGFR TKIs regardless of line of therapy. These results may help in the choice of EGFR-TKI, especially in patients with a poor performance status.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-42 - Real-World Evaluation of Tolerability in Older Adult Patients (≥75 Years Old) with EGFR-mutated NSCLC (ID 13289)
16:45 - 18:00 | Author(s): Ryan N. Walton
- Abstract
Background
NSCLC patients carrying EGFR mutations are diagnosed across a wide age distribution. Although EGFR tyrosine kinase inhibitors (TKIs) are generally well tolerated, there remains a paucity of real-world data on toxicity and health utility scores (HUS) in older patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
A longitudinal observational study evaluated health-related quality of life (HRQoL) using HUS through the EQ-5D questionnaire, and common EGFR-TKI toxicities using PRO-CTCAE in NSCLC outpatients carrying EGFR mutations. Patients were classified into two groups: older (>75 years) and younger (<75 years). Patient characteristics and outcomes were extracted from chart review; patients were classified as having stable or progressive disease according to imaging findings. HUS and PRO-CTCAE results were compared descriptively.
4c3880bb027f159e801041b1021e88e8 Result
Of 240 patients and 774 encounters, 52 patients (22%; comprising 157 encounters) were aged ≥ 75 years. Gender and race were similarly distributed in both age groups: 63% of older patients and 70% of younger (<75 years) were female; 56% of older patients and 53% of younger patients were Asian. Use of gefitinib in older patients was much higher than other drugs: among 147 patients who received gefitinib, 27% (40 patients) were older, compared to 15% (5/33) for osimertinib and 15% (3/20) for erlotinib. Of patients receiving afatinib (n=11) and chemotherapy (n=32), none were ≥ 75 years. The following table describes HUS and PRO-CTCAE results by treatment and age group for stable patients.
Older Adults (≥75 years)
Younger Adults (<75 years)
N
HUS, mean (SD)
PRO-CTCAE*, median [IQR]
N
HUS, mean (SD)
PRO-CTCAE*, median [IQR]
Stable on gefitinib
34
0.83 (0.20)
4.5 [0,16]
77
0.80 (0.15)
4 [0,15]
Stable on osimertinib
5
0.80 (0.23)
13.5 [0,17]
22
0.87 (0.12)
0 [0,13.5]
Stable on erlotinib
3
0.82 (0.08)
0 [0,9]
11
0.80 (0.14)
0 [0,16]
*Higher PRO-CTCAE indicates more severe toxicities/symptoms.
8eea62084ca7e541d918e823422bd82e Conclusion
In a real-world evaluation, patients 75 years or older comprised almost a quarter of all patients with EGFR-mutant advanced NSCLC. Afatinib and chemotherapy were not used at all in this population. Gefitinib was used most commonly, with similar toxicities and health utilities between older and younger patients. Osimertinib and erlotinib were used too infrequently in this study for conclusive age comparisons.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-11 - PRO-CTCAE Toxicities in Advanced NSCLC Patients with EGFR Mutations: A Real World Assessment (ID 12998)
16:45 - 18:00 | Author(s): Ryan N. Walton
- Abstract
Background
The Patient Reported Outcomes of the CTCAE (PRO-CTCAE) tool has not been evaluated in a real-world study of EGFR-mutation positive patients treated with TKIs/chemotherapies. We evaluated its role in capturing clinically-significant toxicities.
a9ded1e5ce5d75814730bb4caaf49419 Method
A longitudinal observational study evaluated common EGFR-TKI toxicities using PRO-CTCAE, measured on a five-point scale (1=no symptoms to 5=very severe symptoms) in outpatients with EGFR-mutated (EGFRm) advanced NSCLC.
4c3880bb027f159e801041b1021e88e8 Result
Toxicity information was collected for 709 follow-up visits (encounters) from 232 patients. Median age was 64 (range:29-96), 161 (69%) were female and 124 (53%) were Asian. 85 (37%) already had brain metastases at first encounter. 485 encounters were observed from patients stable on treatment, and 187 from patients progressing or with documented progression on their current treatment. 24 patients were treated with osimertinib (97 encounters, 97% in second/subsequent-line), 136 with gefitinib (324 encounters, 95% in first line therapy), 42 were receiving other EGFR-TKIs (118 encounters, 53% in second/subsequent-line), and 29 with chemotherapy (73 encounters, 96% second/subsequent-line). The table below summarizes the treatment-related PRO-CTCAE toxicities self-graded as moderate-to-very-severe by EGFRm patients.
Proportion of patients reporting highest grade of toxicity as grade 3-5, by PRO-CTCAE
Gefitinib
Osimertinib
Other EGFR TKI
Chemotherapy
Diarrhea
17%
18%
24%
8%
Constipation
12%
4%
12%
16%
Decreased appetite
10%
7%
14%
26%
Nausea
6%
3%
4%
24%
Vomiting
1%
2%
3%
16%
Fatigue
18%
12%
23%
42%
Numbness and Tingling
6%
7%
10%
16%
Skin Rash
23%
12%
20%
9%
Visual Disorders
(includes dry eye)
4%
0%
3%
4%
Total PRO-CTCAE Score, MEDIAN [IQR]
4 [0,16]
0 [0,15]
6 [0,17]
10 [0,21]
8eea62084ca7e541d918e823422bd82e Conclusion
Osimertinib therapy had the most favorable self-reported toxicity profiles of all the therapies in EGFRm patients, followed by gefitinib. Chemotherapy generated the greatest toxicities. The use of PRO-CTCAE was well-accepted by patients in a clinical setting. This confirms trial data supporting favorable toxicities with osimertinib compared to other therapies for EGFRm NSCLC patients.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 947)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 3
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.15-27 - Patient Characteristics, Treatment Patterns and Survival for Unresectable Stage III NSCLC in Ontario, Canada. (ID 13442)
16:45 - 18:00 | Author(s): Ryan N. Walton
- Abstract
Background
In anticipation of new treatment strategies for unresectable stage III NSCLC, we undertook a retrospective study to determine how these patients have been managed in Ontario, Canada and their survival by treatment approach.
a9ded1e5ce5d75814730bb4caaf49419 Method
Individuals diagnosed with stage III NSCLC between April 1, 2010 and March 31, 2015 were identified in the Ontario Cancer Registry (OCR). Patients with stage III disease were considered to be unresectable if no surgery was undertaken within 3 months of diagnosis. Initial treatments included: radiation (classified as curative or palliative based on treatment intent, body region, dose/fraction, number of fractions); chemotherapy categorized as single or doublet IV chemotherapy, single chemo+RT, doublet chemo+RT, oral targeted therapy. Concurrent chemo+RT (cCRT) was defined as an overlap between chemotherapy and RT, while sequential chemo+RT (sCRT) had no overlap and a 30-day window between treatments. Survival was calculated from date of diagnosis to death.
4c3880bb027f159e801041b1021e88e8 Result
24,729 individuals were diagnosed with NSCLC in Ontario during the study period; 5,243 (21.2%) were stage III and 4,542 (18.4%) were stage III unresectable. Mean age of the unresectable group was 69.7±10.3 years; 54.2% were male. 64.2% of patients were treated within 3 months of diagnosis. The frequency of treatment approach was: cCRT (21.6%), palliative RT (21.3%), curative RT (20.2%), no treatment (19.6%), chemotherapy (11.6%), sCRT (4.9%) and targeted therapy (0.7%). Median survival (IQR) was 2.9 yrs (1.7-4.8) for targeted therapy, 2.0 yrs (1.0-5.5) for cCRT, 1.4 yrs (0.7-3.4) for curative RT, 1.4 yrs (0.7-3.1) for chemotherapy, 1.2 yr (0.6-2.9) for sCRT, 0.6 yrs (0.3-1.2) for palliative RT and 0.5 yrs (0.2-1.2) for no treatment (Figure 1).
Figure 1. Kaplan-Meier survival curves for stage III unresectable lung cancer patients based on treatment type.
8eea62084ca7e541d918e823422bd82e Conclusion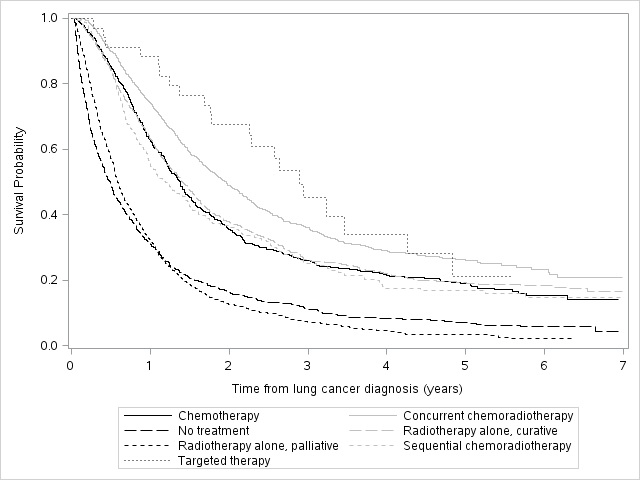
Although cCRT is generally considered standard of care for stage III unresectable NSCLC, patients in Ontario receive various treatment approaches. Survival outcomes vary widely.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.15-28 - Real World Treatment Patterns and Survival of Stage IV Non-Small Cell Lung Cancer (NSCLC) in Ontario, Canada. (ID 13923)
16:45 - 18:00 | Author(s): Ryan N. Walton
- Abstract
Background
The majority of NSCLC patients are diagnosed with stage IV disease. With the development of targeted therapies for advanced NSCLC, it has become important to understand which patients are being treated with systemic therapies and to what benefit.
a9ded1e5ce5d75814730bb4caaf49419 Method
We conducted a longitudinal, population-level study to determine the treatment patterns and survival in patients with stage IV NSCLC in Ontario, Canada between April 1, 2010 and March 31, 2015 from the Ontario Cancer Registry (OCR). Individuals were further identified as having non-squamous disease, and those who received an EGFR-TKI (afatinib, erlotinib, gefitinib) were assumed to be EGFR mutation-positive (EGFR+). Survival was calculated from date of diagnosis to death.
4c3880bb027f159e801041b1021e88e8 Result
24,729 individuals were diagnosed with NSCLC. Approximately half (12,159; 49.2%) had stage IV disease, including 10,103 with non-squamous disease, of whom 508 were categorized as EGFR+. The mean age for the stage IV non-squamous and EGFR+ cohorts were 68.7±11.0 years and 69.1±10.4 years, respectively; 49.3% and 60.8% were female, respectively. The most frequent treatments for stage IV non-squamous patients were palliative radiotherapy (RT) (46.7%) and systemic therapy (14.9%). Patients received no treatment in 26.7% of cases. 75.6% of the EGFR+ cohort received gefitinib, with the majority receiving no subsequent treatment (44.6%). Of EGFR+ patients receiving a second-line treatment, 20.1% received palliative RT and 18.7% received chemotherapy. Mean and median survival times (IQR) for the stage IV non-squamous patients were 0.9±0.0 years and 0.4 (0.2-1.0) years, respectively. Substantial variation in survival was noted by treatment (Figure 1). Mean and median survival times (IQR) for the EGFR+ cohort were 1.9±0.1 years and 1.5 (0.9-3.0) years, respectively.
Figure 1. Kaplan-Meier survival curves for stage IV non-squamous NSCLC patients based on treatment
8eea62084ca7e541d918e823422bd82e Conclusion
Relatively few patients with stage IV non-squamous NSCLC receive any systemic therapy. Survival is generally very poor, but best in the subgroup of EGFR+ patients.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.15-34 - Treatment Patterns and Outcomes of Stage III Non-Small Cell Lung Cancer (NSCLC): Real World Evidence of How our Patients Fare (ID 14011)
16:45 - 18:00 | Author(s): Ryan N. Walton
- Abstract
Background
Most patients withstage III non-small cell lung cancer (NSCLC) develop metastases and succumb to their cancer. New treatment strategies, including concurrent chemo–RT (cCRT) followed by adjuvant immunotherapy, are improving outcomes, but need to be contextualized with real world data. In this study, we described population-based treatment patterns and outcomes for stage III NSCLC in a large Canadian province.
a9ded1e5ce5d75814730bb4caaf49419 Method
Through the provincial cancer registry, patients diagnosed with stage III NSCLC from April 1st 2010 to March 31st 2015 were identified. Using electronic medical records and administrative claims, stage III patients were merged with treatment and survival information. Patient characteristics, treatment patterns, and outcomes were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
6,438 patients were diagnosed with NSCLC, including 1,151 (17.9%) with stage III disease. Median age at diagnosis was 70 years (22–94); 50.2% were male. The majority were stage IIIA (61.2%); the remainder was stage IIIB (36.4%) or unspecified (2.4%). Most patients received palliative RT (32.8%), supportive care until progression (24.8%), or palliative chemotherapy (14.8%) as initial treatments. Relatively few underwent cCRT (11.7%) or trimodality therapy (1.7%). Resection was performed on 14.8% of patients. Within the resected cohort, the majority (47.6%) did not receive further perioperative treatment, while others had surgery as part of trimodality (11.2%) or alongside perioperative chemotherapy (37.1%). Overall, the median OS (mOS) was 13.3 months (0–NR). Initial treatment strategy predicted outcomes (p< 0.05). Patients who underwent cCRT had mOS of 23.8 months (1.1–not reached [NR]). mOS for patients who initially received palliative chemotherapy or RT was 11.1 months (0.3–NR), and 6.2 (0–NR) with supportive care (Figure 1).
Figure 1: Kaplan–Meier curves for stage III NSCLC stratified by treatment class
8eea62084ca7e541d918e823422bd82e Conclusion
Treatment rates for cCRT and trimodality therapy in our cohort appear lower than expected despite evidence supporting the benefits of these strategies. Use of other treatment options was associated with poorer outcomes.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 981)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.15-30 - Treatment Patterns and Survival of Patients with Stage IV Non-Small Cell Lung Cancer (NSCLC) in the Era of Novel Therapies (ID 14042)
12:00 - 13:30 | Author(s): Ryan N. Walton
- Abstract
Background
Treatment strategies for metastatic non-small cell lung cancer (NSCLC) are evolving rapidly. Real–world evidence (RWE) of treatment patterns and outcomes can further our understanding of the impact of novel therapies. In this population-based study, we investigated treatments and outcomes for stage IV NSCLC in a large Canadian province.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients diagnosed with de novostage IV NSCLC from April 1st2010 to March 31st2015 were identified. Baseline characteristics, treatments, and outcomes were analyzed. We classified treatments targeting EGFR, EML4–ALK, and ROS1 as targeted therapy and intravenous checkpoint inhibitors as immunotherapy.
4c3880bb027f159e801041b1021e88e8 Result
A total of 6,438 patients were identified with NSCLC, of whom 3,606 (56%) had de novostage IV disease. The median age of diagnosis was 69 years (range 20–100) and 52.4% were male. The median age among those who received targeted therapy and immunotherapy were 63 (27–90) and 61 (37–72) years, respectively, and 41.8% and 61.8% were male, respectively. First line treatments were: 5.7% targeted agents (n= 204), 1% immunotherapy (n = 1), 19.5% palliative chemotherapy (n= 703), 6.8% palliative radiotherapy (n= 246), and 74.8% received supportive care only (n= 2,698). Most frequent subsequent treatments in 2L included: 30.7% targeted agents (n= 125), 1.7% immunotherapies (n= 7), 67.6% palliative chemotherapy (n= 275), 32.2% palliative radiotherapy (n= 131). Median overall survival (mOS) for the whole cohort was 3.8 months (0–not reached [NR]). MOS with targeted therapies was 18 months (1.4–NR), chemotherapy was 9.4 months (1.1–NR) and supportive care only had a mOS of 2.5 months (0–NR) (Figure 1). About 1.0% of patients (n= 34) received immunotherapy at any line.
8eea62084ca7e541d918e823422bd82e Conclusion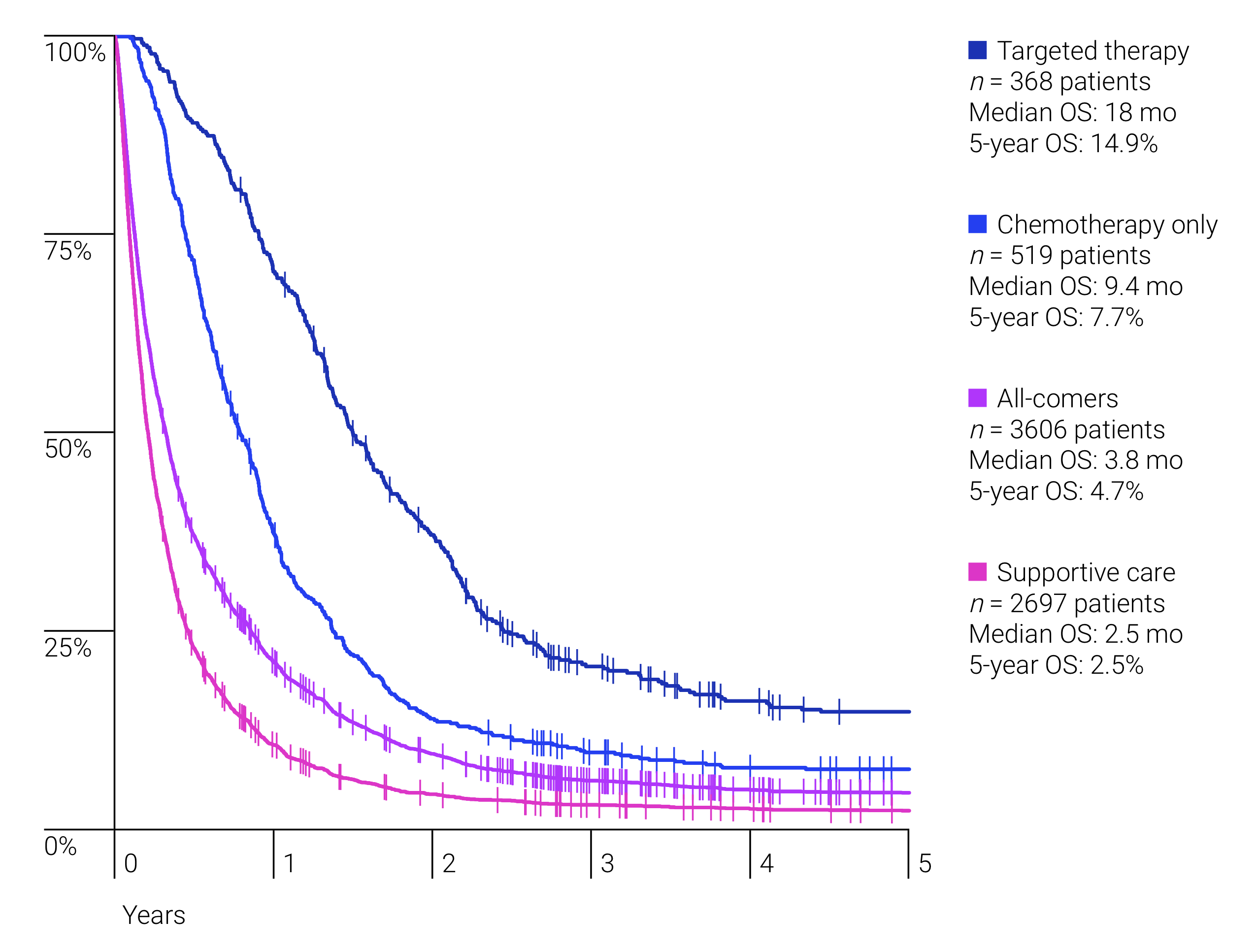
Survival benefit was dependent on type of treatment received, with a trend towards improved survival with newer agents.
6f8b794f3246b0c1e1780bb4d4d5dc53


