Virtual Library
Start Your Search
Mei Sheng Duh
Author of
-
+
MA15 - Colliding Approaches - EGFR and Immunotherapy (ID 916)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 107
-
+
MA15.11 - Real World Biomarker Testing and Treatment Patterns in Patients with Advanced NSCLC Receiving EGFR-TKIs (ID 12705)
14:40 - 14:45 | Author(s): Mei Sheng Duh
- Abstract
- Presentation
Background
In patients who progress on treatment with first- or second-generation EGFR-TKIs, 50–60% will have an EGFR T790M resistance mutation. Osimertinib, a third-generation EGFR-TKI, is FDA approved for use in patients with metastatic EGFR T790M-positive NSCLC and disease progression on or after prior EGFR-TKI therapy, and recently gained additional approval for first-line treatment for patients with EGFR Ex19del/L858R positive advanced NSCLC. We sought to observe how many patients in the real world underwent biomarker testing on progression and subsequently received osimertinib, when T790M positive.
a9ded1e5ce5d75814730bb4caaf49419 Method
Flatiron Health EHR-derived database was used to identify adult patients with NSCLC treated with a first- or second-generation EGFR-TKI from 11/2015–09/2017, with the start of first EGFR-TKI defined as the index date. Patients were stratified by EGFR-TKI use as a first (1L) or later line (2L+) treatment. EGFRm status, including T790M testing and subsequent treatments received after initiating first- or second-generation EGFR-TKI, were described. Chart review was conducted on patients who received a subsequent therapy to confirm disease progression.
4c3880bb027f159e801041b1021e88e8 Result
Patients in this study (n=782; 1L: n=435; 2L+: n=347) had a median age of 69 years, 64% were female, 56% were white, 87% were seen in the community, with a median follow-up of 307 days. During the study period, 30% (235/782; 1L: 96/435 [22%]; 2L+: 139/347 [40%]) of patients died without receiving a subsequent therapy, and 38% (294/782; 1L: 160/435 [37%]; 2L+: 134/347 [39%]) received subsequent therapies. From post-index date to initiation of subsequent therapies, 30% (88/294; 1L 63/160 [39%]; 2L+ 25/134 [19%]) of patients were tested for EGFR mutations including T790M. Among patients who received subsequent therapies, treatments included chemotherapies (1L=23%; 2L+=37%), immunotherapies (1L=16%; 2L+=43%), and targeted therapies (1L=64%; 2L+=30%). On progression, 25% (1L 40/160) and 5% (2L+ 7/134) of the patients received osimertinib. Of those tested for EGFR mutation post-index date (n=88), 28% (n=25) were positive for T790M and 96% of these (n=24) received osimertinib. Most patients (251/294 [85%]; 1L: 136/160 [85%]; 2L+: 115/134 [86%]) on subsequent therapies were confirmed to have disease progression at chart review.
8eea62084ca7e541d918e823422bd82e Conclusion
In this study, a third of patients were tested for subsequent EGFR mutations including EGFR T790M following treatment with first- or second-generation EGFR-TKI. Of these, a third were positive for T790M and nearly all of the T790M positive patients received osimertinib. These findings highlight low rates of biomarker testing at progression, and the need for optimal treatments that maximize benefits for patients with EGFRm NSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P2.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 964)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.15-26 - Rates and Economic Burden of Adverse Events in Patients With Metastatic NSCLC Treated with EGFR-TKIs (ID 13643)
16:45 - 18:00 | Author(s): Mei Sheng Duh
- Abstract
Background
Trials of first- and second-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have reported severe adverse events (SAEs) in 6%–49% of patients with EGFR-mutant (EGFRm) metastatic non-small cell lung cancer (mNSCLC). This study describes the real-world rates and incremental cost of AEs in this population.
a9ded1e5ce5d75814730bb4caaf49419 Method
Adults with mNSCLC treated with EGFR-TKIs as first-line therapy (first dispensing defined as the index date) with ≥1 lung cancer diagnosis code, no claim for a lung surgery, and no administration of other NSCLC therapies during 3 months pre-index date were identified from the IQVIATM Real-World Data Adjudicated Claims – US database (Q2/2012–Q1/2017). The select AEs identified from prescribing information of EGFR-TKIs were skin and ocular disorders, interstitial lung disease (ILD), diarrhea, microangiopathic hemolytic anemia, gastrointestinal perforation, cardiac and cerebrovascular events, renal failure, and hepatotoxicity. AE rates per 1,000 patient-years and healthcare cost per-patient-per-month (PPPM) were calculated during first-line therapy. Multivariate linear regression was used to assess cost differences (CD) for patients with and without AEs, adjusting for baseline characteristics. All AEs (outpatient and hospitalization claims) and a subgroup of SAEs (hospitalization claims) were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
Among 1,646 patients, 86.1% were treated with erlotinib, 12.1% with afatinib, and 1.0% with gefitinib. During first-line therapy, 549 patients had ≥1 acute AE (33.4%, 870.9 per 1,000 patient-years) and 200 patients had ≥1 acute SAE (12.2%, 220.1 per 1,000 patient-years). Skin and ocular disorders (17.6%, 482.9 per 1,000 patient-years) and ILD (4.5%, 139.6 per 1,000 patient-years) had the highest rate among all AEs and SAEs, respectively (Figure). Patients with AEs had higher PPPM healthcare costs than patients without AEs (all AEs: CD=$1,079, p<0.001; SAEs: CD=$4,700, p<0.001).
8eea62084ca7e541d918e823422bd82e Conclusion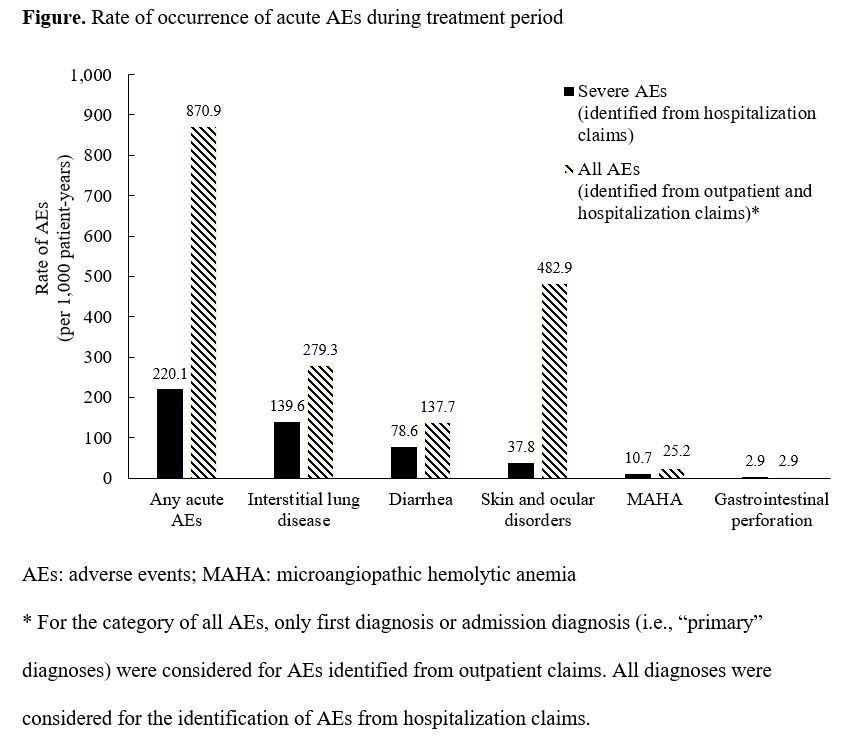
Over one-tenth of patients suffered from SAEs, resulting in a sizeable economic burden. EGFR-TKIs with more favorable safety profiles may reduce substantial healthcare costs in this patient population.
6f8b794f3246b0c1e1780bb4d4d5dc53


