Virtual Library
Start Your Search
Frank Aboubakar Nana
Author of
-
+
OA09 - Prevention and Cessation (ID 909)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Prevention and Tobacco Control
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 15:15 - 16:45, Room 205 BD
-
+
OA09.06 - Molecular Alterations and Estimated Indoor Radon in NSCLC Patients from the French National Cancer Institute Registry: Radon France Study (ID 14168)
16:10 - 16:20 | Author(s): Frank Aboubakar Nana
- Abstract
- Presentation
Background
Radon is a radioactive gas, considered the leading cause of lung cancer in non-smokers. We assessed the correlation between the radon exposure areas in France and the molecular alterations nationally registered in non-small cell lung cancer (NSCLC) patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively collected all NSCLC tested for EGFR, BRAF, HER2 and KRAS mutations (m) and ALK and ROS1 rearrangements (r) on the 28 French Plateform led by INCa (French National Cancer Institute). The prevalence of molecular alterations by region was correlated to the indoor radon risk area based on the official French (Institut de Radioprotection et de Sûreté Nucléaire, INSN, France). Paris and its region Ile-de-France were not included in this analysis due to its high rate of patients that are native from other regions.
4c3880bb027f159e801041b1021e88e8 Result
116.424 NSCLC were included. Overall, KRAS was positive in 27,7% (27.314/98.522), EGFR in 11,27% (13.125/116.424), ALK in 3,2% (2.928/91.291), BRAF in 2,3% (2.419/105.919), ROS1 in 1,12% (373/33.222) and HER2 in 0,8% (816/97.749) of all cases.
We stratified the French regions in 3 areas based on their exposure to radon: high (Auvergne-Rhône-Alpes, Bretagne, Normandie, Pays de la Loire), intermediate (Bourgogne-Franche-Comté, Nouvelle Aquitaine, Occitanie, Provence-Alpes-Cote-d'Azur) and low explosure (Centre Val-de-Loire, Grand Est, Hauts de France). The prevalence of driver alterations (EGFR, BRAF, HER2 and ROS1 were significantly higher in high exposure area. The prevalence of KRAS mutations was significantly higher in low exposure area.
8eea62084ca7e541d918e823422bd82e ConclusionLow risk
Intermediate
High
P
EGFR mutation
1962 (10%)
4338 (11%)
4176 (11.4%)
<0.0001
ALK rearrangement
577 (3.3%)
1019 (3%)
896 (3%)
0.35
BRAF mutation
327 (1.8%)
830 (2.4%)
692 (2.4%)
0.0001
HER2 mutation
109 (0.6%)
266 (0.9%)
252 (0.8%)
0.01
ROS1 rearrangement
61 (0.9%)
133 (0.9%)
126 (1.3%)
0.005
KRAS mutation
4717 (29.8%)
9215 (28.2%)
7895 (27%)
<0.0001
Molecular drivers*
3037 (3.9%)
6587 (4.4%)
6142 (4.4%)
<0.0001
* EGFR, BRAF & HER2 mutations, ALK & ROS1 rearrangements; KRAS mutation excluded.
NSCLC molecular alterations that are linked to low tobacco consumption were higher in the French region with high radon exposure. Role of the radon in lung cancer carcinogenesis of specific molecular subtypes should be further explored.
6f8b794f3246b0c1e1780bb4d4d5dc53Information from this presentation has been removed upon request of the author.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-07 - Immune-Related Pneumonitis in NSCLC Patients Treated with Immune Checkpoint Inhibitors (ICI): Impact of Previous Thoracic Radiotherapy (ID 12805)
16:45 - 18:00 | Author(s): Frank Aboubakar Nana
- Abstract
Background
Pneumonitis is a potentially lethal side effect of immune checkpoint inhibitors (ICI), occurring in 1–5% of patients enrolled in clinical trials. Little is known about the interactions between ICI and previous thoracic radiation. This is the aim of the present study.
a9ded1e5ce5d75814730bb4caaf49419 Method
Between December 2012 and November 2017, 318 consecutive non-small cell lung cancer (NSCLC) patients received ICI in our Institution and their charts were retrospectively analyzed. Primary endpoint was to determine whether previous radiotherapy had an effect on pulmonary toxicity. Pulmonary toxicity was retrospectively assessed by Common Terminology Criteria for Adverse Events version 4.0.
4c3880bb027f159e801041b1021e88e8 Result
Median follow-up was 32.8 months [95%CI: 5-190]. Median age at the start of ICI was 63 years. 205 patients (64,5%) were males, 103 (32,4%) smokers and 250 (78,6%) with PS ≤1; 206 (64,8%) had adenocarcinoma and 76 (23,9%) squamous; 79 (24,8%) were KRAS mutated, 18 (5,5%) EGFR mutated and 5 (1,6%) ALK positive. PDL1 was ≥ 1% by immunohistochemistry in 86 (27%), negative in 37 (11,6%) and unknown in 196 (61,3%) patients. ICI treatment was median 3rd line (range: 1-12), 89,4% monotherapy PD-(L)1 inhibition.
72 patients (22,6%) received a thoracic RT: 62 out of the 72 RT patients (87,5%) were irradiated with a curative intent. 53 patients (73,6% of the RT patients) received thoracic 3D-conformal RT or intensity modulated RT (normo- or mildly hypofractionated), whereas 9 received SBRT.
16,7% of the RT patients (12/72) showed a G1-4 immune-related pneumonitis (with a G=>3 of 11,1%), whereas for never-irradiated patients the G1-3 rate of immune-related pneumonitis was 2,4% (6/246), with only 1 G3 toxicity observed and no G>4 (t-test, p 0,001).
Median interval between the onset of the immune-related pneumonitis and the end of the RT was 22,4 months.
8eea62084ca7e541d918e823422bd82e Conclusion
NSCLC patients treated with ICI may be at higher risk of developing immune-related pneumonitis when previously treated with curative-intent thoracic RT.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-18 - Immunosenescence Correlates with Progression upon PD-(L)-1 Blockade (IO) in Advanced Non-Small Cell Lung Cancer (aNSCLC) Patients. (ID 14074)
16:45 - 18:00 | Author(s): Frank Aboubakar Nana
- Abstract
Background
Immunosenescence is a progressive remodeling of immune functions with a multifactorial etiology (i.e. aging, chronic inflammation, cancer). Although a CD28-CD57+KLRG1+ phenotype on peripheral T-lymphocytes is a potential hallmark of immunosenescence, the characterization of such phenotype in IO-treated NSCLC patients and the correlation with clinical characteristics and benefit from immunotherapy are unknown.
a9ded1e5ce5d75814730bb4caaf49419 Method
A senescent immune phenotype (SIP) defined as a percentage of circulating CD8+CD28-CD57+KLRG1+ T-lymphocytes was assessed by flow cytometry (FC) on fresh blood samples from IO-treated aNSCLC patients (03/2017–04/2018). A log-rank maximization method was used to identify a SIP cut-off level and dichotomize patients accordingly. The objective was to correlate SIP with clinical characteristics and RECIST response by univariate logistic regression analysis.
4c3880bb027f159e801041b1021e88e8 Result
39 aNSCLC patients were evaluable for SIP before IO: 38% ≥ 65 years, 87% non-squamous, 38% KRAS mutated, 54% with PD-L1 expression ≥1%, 13% chemotherapy naïve. Among 30 patients evaluable for IO response, 53% had progression (PD), 27% stability (SD), 20% partial response (PR). Median PFS was 1.9 months (95% CI 1.5; 2.5). OS was not calculated due to the short follow-up [6 months (95% CI 4-11)]. SIP (% CD28-CD57+KLRG1+) median value on circulating CD8+ lymphocytes was 15.26% (min 1.87%, max 56.28%). Overall, 13 (33%) of 39 patients had >22.25% CD8+ lymphocytes with a CD28-CD57+KLRG1+ phenotype, being classified as SIP+. SIP status did not correlate with age, IO-baseline patients’ characteristics or chemotherapy exposure. Among patients evaluable for IO response, only 1 (10%) of 10 SIP+ experienced disease control (PR/SD), compared to 13 (65%) of 20 SIP- patients; similarly, PD rate was significantly higher in SIP+ compared to SIP- patients (90% vs 35%, p=0.007) (Figure).
8eea62084ca7e541d918e823422bd82e Conclusion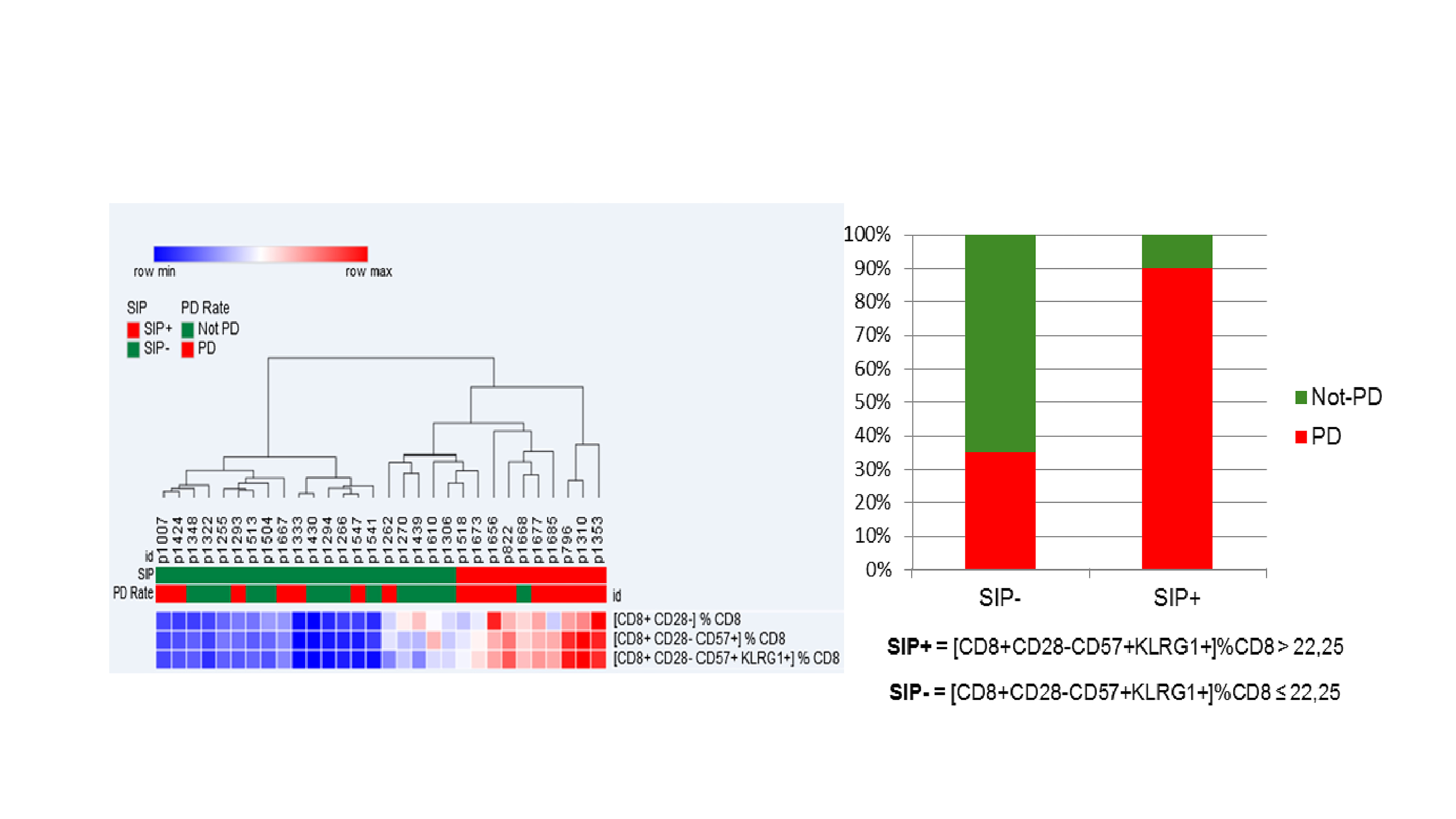
Immunosenescence, monitored by FC measurement of 3 surface molecules on circulating CD8 + lymphocytes, is observed in 33% of aNSCLC patients, is independent of age and correlates with lower IO disease control rate.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.13 - Targeted Therapy (Not CME Accredited Session) (ID 962)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.13-24 - Prospective Efficacy of Osimertinib in Circulating Tumour DNA (ctDNA) T790M-Mutant NSCLC Patients (ID 14031)
16:45 - 18:00 | Author(s): Frank Aboubakar Nana
- Abstract
Background
Liquid biopsy circulating tumor DNA (ctDNA) analysis in advanced EGFR-mutant NSCLC patients is an approved tool for molecular profiling and disease surveillance when tissue is not available. Long-term efficacy of osimertinib in patients with the T790M resistance mutation positive detected only by ctDNA (without tissue information) has not been fully validated.
a9ded1e5ce5d75814730bb4caaf49419 Method
In a prospective study, EGFR-mutant advanced NSCLC patients with acquired resistance to EGFR TKI, in whom a repeat tissue biopsy was not feasible, were assessed for ctDNA T790M mutational status using InVisionSeqTM. T790M-positive NSCLC patients received osimertinib (80 mg daily; extended access program or approval) at RECIST progression. The objectives were to assess: proportion of patients with acquired ctDNA-T790M positive; overall survival (OS) of the overall EGFR-mutant population as well as OS comparison for T790M +ve/-ve. Also, for those T790M-positive NSCLC patients who received osimertinib in a real world data we assessed: response rate (RR) according to RECIST 1.1 by investigator and progression free survival (PFS), calculated from the date of osimertinib initiation until the date of progression or death (whichever came first), or the date of last follow-up are also reported.
4c3880bb027f159e801041b1021e88e8 Result
We recruited 82 patients (71% female, median age 64 years, 72% Del19 EGFR mutation, 71% never-smokers). The ctDNA T790M mutation was detected in 55% (N=45) of NSCLC patients. Median OS of EGFR-mutant population was 38.2 months (mo.). According to T790M status, median OS was 41.2 months and 30.4 mo. for T790M-positive and T790M-negative NSCLC patients, respectively. Both cohorts had already received a median of 3 previous treatment lines. In 40 T790M-positive NSCLC patients who receive osimertinib, RR was 55% (PR: 55%, SD 27.5% and PD: 12.5%) and median PFS of 8.5 mo. Median OS on osimertinib among 10 patients with brain and/or leptomeningeal metastases at baseline was of 13.4 months.
8eea62084ca7e541d918e823422bd82e Conclusion
In patients with acquired resistance to first- or second-generation EGFR TKIs, ctDNA T790M detection by InVisionSeq™ is equivalent to what has been reported in tissue biopsy. Osimertinib has clinical benefit in patients for which the T790M resistance mutation is detected only through a liquid biopsy procedure.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 978)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.12-11 - Association of the Lung Immune Prognostic Index (LIPI) with Outcomes for Immune Checkpoint Inhibitors in Diffuse SCLC Patients (ID 14200)
12:00 - 13:30 | Author(s): Frank Aboubakar Nana
- Abstract
Background
Pretreatment LIPI (Lung Immune Prognostic Index), based on derived NLR (neutrophils/[leucocytes-neutrophils] ratio) and lactate dehydrogenase (LDH) has been associated with outcomes for immune checkpoint inhibitors (ICI) in advanced NSCLC patients. We tested whether LIPI has the same role in diffuse small cell lung cancer (SCLC) patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
Baseline dNLR and LDH and clinical data were retrospectively collected in SCLC patients, treated with ICI (PD1 inhibitor, PDL1 inhibitors +/- CTLA4 inhibitor) from April 2014 to Jan. 2018 (N=66) from 6 European centers. LIPI was calculated combining dNLR and LDH, stratifying 3 risk groups: good (dNLR<3+LDH<upper limit of normal (ULN), intermediate (dNLR>3 or LDH>ULN), poor (dNLR>3+LDH>ULN). The primary endpoint was overall survival (OS), and secondary endpoint was progression-free survival (PFS).
4c3880bb027f159e801041b1021e88e8 Result
Fifty-three patients (80%) were males, 58 (88%) smokers and all patients had PS ≤1, with median age 63 years (41-82). PDL1 was ≥ 1% by immunohistochemistry in 6 patients, and unknown in 60 patients. The median of prior lines was 1 (0-6). Platinum-based therapy was the prior line in 63 (95%) patients, with ORR of 88%. The median PFS and OS with ICI were 2.7 months (m) [95% CI 1.87-4.43] and 10.3 m [95% CI 5.8-12.6]. dNLR was greater than 3 in 16 (25%) and LDH> Upper Limit of Normal (ULN) in 33 (50%) patients. Based on both, LIPI stratified the population in 3 groups: 26 patients as good (40%), 29 (45%) as intermediate and 10 (15%) as poor LIPI risk groups. LIPI was an independent factor for OS (HR 2.77, 95% CI 1.07-7.14, P=0.03) and PFS (HR 3.13, 1.37-7.16, P=0.01). Median OS for good, intermediate, and poor risk groups were 11.4 m [95% CI 5.5-27.3], 11 m [95% CI 6.8-not-reached (NR)] and 2.3 m [95% CI 0.7-NR], respectively (P=0.004). Median PFS for good, intermediate, and poor risk groups were 3 m [95% CI 1.9-12.6], 2.8 m [95% CI 1.6-6.0 and 1.2 m [95% CI 0.47-NR], respectively (P=0.004).
8eea62084ca7e541d918e823422bd82e Conclusion
Baseline LIPI poor risk group is associated with poor outcomes for ICI in diffuse SCLC patients. LIPI effect in a validation cohort is currently evaluated.
6f8b794f3246b0c1e1780bb4d4d5dc53


