Virtual Library
Start Your Search
William Kenneth Evans
Author of
-
+
EX04 - Mini Oral Abstract Session - MA08.06, MA18.02, MA19.02, MA20.11 (ID 1006)
- Event: WCLC 2018
- Type: Exhibit Showcase
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 09:55 - 10:25, Exhibit Showcase Theater
-
+
EX04.02 - The Impact of Treatment Evolution in NSCLC (iTEN) Model: Development and Validation (ID 13468)
10:00 - 10:05 | Author(s): William Kenneth Evans
- Abstract
Background
Background: The iTEN model was developed to estimate the survival impact of new treatments for advanced NSCLC (aNSCLC) patients. The structure and key assumptions of the iTEN model and outputs validated against published real-world survival data are presented.
a9ded1e5ce5d75814730bb4caaf49419 Method
Methods: The iTEN model is a discrete event simulation of aNSCLC patients’ treatment plans. Individual patient characteristics (histology, molecular subtypes (EGFR, ALK, ROS1, BRAF, PD-L1), and performance status) are generated by random sampling from Canadian prevalence estimates. All Health Canada approved agents for treatment of aNSCLC are included. Simulated patients start on first-line therapy and move to subsequent lines of therapy in modelled progression events. Up to six-lines of therapy can be included. Time-of-event for progression or death for each patient is calculated based on random probabilities and progression-free survival (PFS) and overall survival (OS) curves modelled from published clinical trials. For example, a simulated ALK+ patient might receive first-line crizotinib, followed by second-line ceritinib and BSC, based on PFS/OS data from PROFILE 1014 and ASCEND-5. Predicted OS is calculated as the cumulative time spent on active therapy and BSC. PFS/OS data were extrapolated using best practices. Treatment on previous therapies was assumed to have no impact on the efficacy of subsequent therapies. Model survival predictions were validated against published real-world estimates from the Ontario Cancer and Austrian (TYROL) registries, by reproducing the same treatment mix in the simulated patients as in the publications.
4c3880bb027f159e801041b1021e88e8 Result
Results: iTEN estimated two- to five-year survival rates were similar to those reported by the Ontario Cancer and TYROL registries.
8eea62084ca7e541d918e823422bd82e Conclusion
Conclusions: While further analyses are required, the iTEN model produces survival estimates comparable to published real-world data; therefore, the iTEN model may be a valid tool to estimate aNSCLC patient survival.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
MA02 - Improving Outcomes for Patients with Lung Cancer (ID 895)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 201 BD
-
+
MA02.12 - Discussant - MA 02.10, MA 02.11 (ID 14577)
11:45 - 12:00 | Presenting Author(s): William Kenneth Evans
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA18 - Modelling, Decision-Making and Population-Based Outcomes (ID 920)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment in the Real World - Support, Survivorship, Systems Research
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 201 F
-
+
MA18.03 - How in the Real World Are Lung Cancer Patients Treated? The Ontario, Canada Experience (ID 13772)
13:35 - 13:40 | Presenting Author(s): William Kenneth Evans
- Abstract
- Presentation
Background
Clinical trials define treatment recommendations but how patients are actually treated in the real world is poorly understood. The Canadian Partnership Against Cancer has developed a model of lung cancer (LC) management (OncoSim-lung) based on clinical trials data and expert advice. To credibly project the future clinical and economic impacts of cancer control measures using OncoSim, the model has been refined using real-world data.
a9ded1e5ce5d75814730bb4caaf49419 Method
Treatment data by histology and stage were extracted from the Ontario Cancer Registry for LC cohorts diagnosed in 2010 and 2013. All incident cases that satisfied the IARC rule of a new primary were included. Missing or unknown stage cases were excluded. Clinical pathways were validated by oncologists from different disciplines across Canada.
4c3880bb027f159e801041b1021e88e8 Result
The 2013 cohort included 8,086 staged LC: NSCLC (n=7,143) Stage I 18.7%, II 8%, III/IIIa 11.4%, IIIb 4.9% IV 56.8%; SCLC (n= 943) limited 67.7%, extensive 32.3%. Of 1340 stage I NSCLC patients, 61% underwent surgery; 39% had no surgery and one third of these had no active treatment (NAT). 55% of those not receiving surgery underwent radical radiotherapy and 6% had palliative radiotherapy. Of 579 patients with stage II NSCLC, 60% underwent surgery and 47% of these received adjuvant chemotherapy; 40% had no surgery and 22% of these had NAT. Radical radiotherapy, radiotherapy plus chemotherapy or palliative radiotherapy were given in 33%, 19% and 18% of non-surgical cases, respectively. Of 813 stage III/IIIa patients, only 26% underwent surgery, 41% of whom received adjuvant chemotherapy or postoperative radical radiotherapy (16%); 13% received trimodality treatment. Of the 75% of Stage III not receiving surgery, 26% had NAT and 21% had palliative radiotherapy alone. Of those receiving active treatment, 20% received combined chemo +radiotherapy and 13% each had chemotherapy alone or radical radiotherapy alone. Of 356 stage IIIb patients, 17% had NAT, 28% received palliative radiotherapy and only 30% had chemo + radical radiotherapy. 18% had chemo alone. Of 4055 stage IV NSCLC, 47% had NAT, 24% received chemotherapy alone and 23% had palliative radiotherapy only. Of those who received first-line chemotherapy (n= 1059), 47% received second line chemotherapy and of those, 37% received third line therapy.
8eea62084ca7e541d918e823422bd82e Conclusion
Compared to prior expert opinion, there was a higher use of radiotherapy for early stage disease, a lower frequency of chemo-radiotherapy in Stage III disease and a higher frequency of NAT across all stages of disease.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA18.07 - Awareness of the Harms of Continued Smoking Among Lung Cancer (LC) Survivors (ID 12024)
14:05 - 14:10 | Author(s): William Kenneth Evans
- Abstract
- Presentation
Background
Continued smoking after a LC diagnosis is associated with poorer cancer outcomes including increased risk of treatment-related side-effects, reduced treatment efficacy and poorer prognosis. Smoking cessation is an integral part of LC survivorship by improving both cancer and non-cancer outcomes. To enhance survivorship education, clinicians should understand patient awareness of the harms of continued smoking.
a9ded1e5ce5d75814730bb4caaf49419 Method
LC survivors from Princess Margaret Cancer Centre, Toronto (2014-2017) were surveyed with respect to self-awareness of the harms of continued smoking on cancer-related outcomes. Univariable and multivariable logistic regression models assessed factors associated with awareness and whether awareness was associated with cessation among current smokers at diagnosis.
4c3880bb027f159e801041b1021e88e8 Result
Of 553 patients, 181 were lifetime never-smokers. Among those smoking during the peri-diagnosis period (n=177), 65% quit after diagnosis. Among all, few patients were aware that smoking negatively impacts treatment-related outcomes [complications from cancer surgery (only 41% aware), radiation side-effects (30%), quality-of-life on chemotherapy (44%) and treatment efficacy (36%)]; half were aware that smoking negatively impacts cancer prognosis (51% aware) and risk of developing second primaries (50%). Compared to ex-smokers/never-smokers at diagnosis, current smokers at diagnosis were less aware of the impact of smoking on radiation side-effects (22% vs 31% aware, P=0.01), prognosis (44% vs 55%, P=0.02) and risk of second primaries (42% vs 55%, P=0.007). Among sociodemographic variables, only those speaking English at home were consistently found more likely unaware that smoking negatively impacts these outcomes (ORs=1.52-2.20, P<0.04). Patients with early stage disease were more likely unaware that smoking negative impacts radiation side-effects (OR=1.60, 95%CI[1.09-2.35], P=0.02); while patients on curative treatment (OR=1.53[1.08-2.17], P=0.02) and those exposed to second-hand smoke (SHS) were more likely unaware that smoking impacts quality-of-life on chemotherapy (OR=1.64[1.05-2.58], P=0.03). Exposure to SHS, treatment intent and stage were not associated with awareness of impact on prognosis or second primaries (P>0.11). Among smokers in the peri-diagnosis period, awareness of the impact of smoking on surgical complications (aOR=2.09 [0.96-4.54], P=0.06), quality-of-life while receiving chemotherapy (aOR=2.60[1.17-5.79], P=0.02) and on treatment efficacy (aOR =2.24[0.97-5.20], P=0.06) were each associated with subsequent quitting, adjusted for marital status, pack-years, self-rated health and SHS exposure.
8eea62084ca7e541d918e823422bd82e Conclusion
Many LC patients are unaware of the harms of continued smoking on cancer outcomes, particularly those smoking at diagnosis. Awareness of some of these outcomes was associated with subsequent tobacco cessation. Patient education on the health benefits of smoking cessation may increase quit rates and improve outcomes for LC patients.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA09 - Prevention and Cessation (ID 909)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Prevention and Tobacco Control
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 15:15 - 16:45, Room 205 BD
-
+
OA09.01 - 5As to 3As: Evolution of the Systematic Approach to Smoking Cessation in Ontario’s Regional Cancer Centres (ID 14066)
15:15 - 15:25 | Presenting Author(s): William Kenneth Evans
- Abstract
- Presentation
Background
Smoking is responsible for approximately 30% of all cancer deaths in Canada, and more than 85% of lung cancer cases. Cancer patients who continue to smoke experience decreased treatment efficacy and safety, increased toxicities, greater risk of cancer recurrence and second primaries, poorer quality of life, and decreased survival. Evidence suggests that quitting smoking after diagnosis can significantly reduce these adverse effects. In 2012, Cancer Care Ontario (CCO) introduced a Framework for Smoking Cessation to be implemented across the province’s 14 Regional Cancer Centres (RCCs). In 2017, the Framework was revised from a 5As (Ask, Advise, Assess, Assist, Arrange) to a 3As (Ask, Advise, Act) brief intervention model.
a9ded1e5ce5d75814730bb4caaf49419 Method
The transition to a 3As model was based on emerging evidence, feedback from CCO’s Smoking Cessation Advisory Committee and Regional Smoking Cessation Champions, as well as learnings from a preliminary program evaluation. The revised Framework recommended an “opt-out” approach to referring smokers to cessation services. Following an environmental scan and site visit with each RCC to assess the current state, site-specific action plans were developed to promote alignment with the revised Framework. Action steps were given priority ratings in the areas of data capture, referrals, and resources. Two phone calls were held with each RCC to monitor progress on action plan implementation. Knowledge translation resources were created to support healthcare providers’ uptake of the 3As model.
4c3880bb027f159e801041b1021e88e8 Result
Smoking cessation interventions are often perceived by health care providers as time-consuming; the 3As model made the intervention briefer but no less effective. Over 3,000 knowledge translation resources were distributed to support healthcare providers working directly with cancer patients, including pocket cards and posters with suggested scripts. While the revised Framework officially launched in April 2018, early adopters of the 3As model and opt-out approach have seen improved performance on the Accepted a Referral indicator (proportion of smokers who accepted a referral to cessation services). In 2017, one RCC’s rate tripled from 10.1% to 30.9% in 6 months, while another improved from 13.2% to 36.9% in the same period.
8eea62084ca7e541d918e823422bd82e Conclusion
To improve program effectiveness, CCO’s smoking cessation initiative transitioned from a 5As to a 3As model and an opt-out referral process. Frontline staff have indicated a willingness to adopt the simplified approach, and early results show a promising increase in the number of smokers who are connected to smoking cessation services.
6f8b794f3246b0c1e1780bb4d4d5dc53Information from this presentation has been removed upon request of the author.
-
+
OA09.02 - Acceptance of Smoking Cessation Services in Cancer Care Ontario’s Lung Cancer Screening Pilot for People at High Risk (ID 13032)
15:25 - 15:35 | Presenting Author(s): William Kenneth Evans
- Abstract
- Presentation
Background
Participation in lung cancer screening can be a teachable moment for smoking cessation. Current smokers who attend for lung screening may also be motivated to quit. In June 2017, Cancer Care Ontario launched organized lung cancer screening at 3 pilot sites in Ontario with smoking cessation embedded in the screening pathway. Participants are recruited through primary care providers and public-facing messaging.
a9ded1e5ce5d75814730bb4caaf49419 Method
Smoking cessation services (SCS) are offered to all current smokers (anyone who smoked a cigarette in the past 30 days) interacting with the pilot. Individuals found ineligible for screening are offered a direct referral to the Canadian Cancer Society’s Smokers’ Helpline. Screen-eligible individuals are scheduled for smoking cessation counselling during their baseline low-dose computed tomography (CT) appointment, using an opt-out approach. Hospital-based SCS are provided by trained counsellors and consist of 10 minutes (minimum) of behavioural counselling, a recommendation or prescription for pharmacotherapy, and arrangements for proactive follow-up. The proportions of current smokers who accept referral to SCS and who attend hospital-based smoking cessation counselling are being monitored throughout the pilot. A participant satisfaction survey is completed after the screening appointment (if applicable). Data on quit rates, quit attempts, heaviness of smoking and relapse among screening participants is being captured.
4c3880bb027f159e801041b1021e88e8 Result
Between June and October 2017, 50% of the 1241 individuals who underwent risk assessment to determine eligibility for screening were current smokers. Of the 808 individuals eligible for screening, 63% were current smokers: 52% were male, (age 55-64, 61%; 65-74, 39%), 55% had a high school education or less. 27% of ineligible individuals were current smokers. 83% of all current smokers (regardless of screen-eligibility) accepted a referral to SCS. Of screen-eligible current smokers, 89% accepted hospital-based cessation counselling; 88% of those who had a baseline low-dose CT in the reporting period attended a hospital-based counselling session. 93% of survey respondents (response rate 56%) reported being satisfied with the smoking cessation counselling they received.
8eea62084ca7e541d918e823422bd82e Conclusion
Acceptance of SCS by current smokers in Cancer Care Ontario’s lung cancer screening pilot is very high. A large majority of screened current smokers have attended a hospital-based counselling session, and satisfaction with this service was high. These findings suggest that an opt-out approach is acceptable to individuals motivated to attend a lung screening program. The final pilot evaluation in spring 2020 will evaluate the success of the smoking cessation initiative by assessing quit attempts, quit rates and relapse among screening participants.
6f8b794f3246b0c1e1780bb4d4d5dc53Information from this presentation has been removed upon request of the author.
-
+
P1.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 947)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.15-27 - Patient Characteristics, Treatment Patterns and Survival for Unresectable Stage III NSCLC in Ontario, Canada. (ID 13442)
16:45 - 18:00 | Author(s): William Kenneth Evans
- Abstract
Background
In anticipation of new treatment strategies for unresectable stage III NSCLC, we undertook a retrospective study to determine how these patients have been managed in Ontario, Canada and their survival by treatment approach.
a9ded1e5ce5d75814730bb4caaf49419 Method
Individuals diagnosed with stage III NSCLC between April 1, 2010 and March 31, 2015 were identified in the Ontario Cancer Registry (OCR). Patients with stage III disease were considered to be unresectable if no surgery was undertaken within 3 months of diagnosis. Initial treatments included: radiation (classified as curative or palliative based on treatment intent, body region, dose/fraction, number of fractions); chemotherapy categorized as single or doublet IV chemotherapy, single chemo+RT, doublet chemo+RT, oral targeted therapy. Concurrent chemo+RT (cCRT) was defined as an overlap between chemotherapy and RT, while sequential chemo+RT (sCRT) had no overlap and a 30-day window between treatments. Survival was calculated from date of diagnosis to death.
4c3880bb027f159e801041b1021e88e8 Result
24,729 individuals were diagnosed with NSCLC in Ontario during the study period; 5,243 (21.2%) were stage III and 4,542 (18.4%) were stage III unresectable. Mean age of the unresectable group was 69.7±10.3 years; 54.2% were male. 64.2% of patients were treated within 3 months of diagnosis. The frequency of treatment approach was: cCRT (21.6%), palliative RT (21.3%), curative RT (20.2%), no treatment (19.6%), chemotherapy (11.6%), sCRT (4.9%) and targeted therapy (0.7%). Median survival (IQR) was 2.9 yrs (1.7-4.8) for targeted therapy, 2.0 yrs (1.0-5.5) for cCRT, 1.4 yrs (0.7-3.4) for curative RT, 1.4 yrs (0.7-3.1) for chemotherapy, 1.2 yr (0.6-2.9) for sCRT, 0.6 yrs (0.3-1.2) for palliative RT and 0.5 yrs (0.2-1.2) for no treatment (Figure 1).
Figure 1. Kaplan-Meier survival curves for stage III unresectable lung cancer patients based on treatment type.
8eea62084ca7e541d918e823422bd82e Conclusion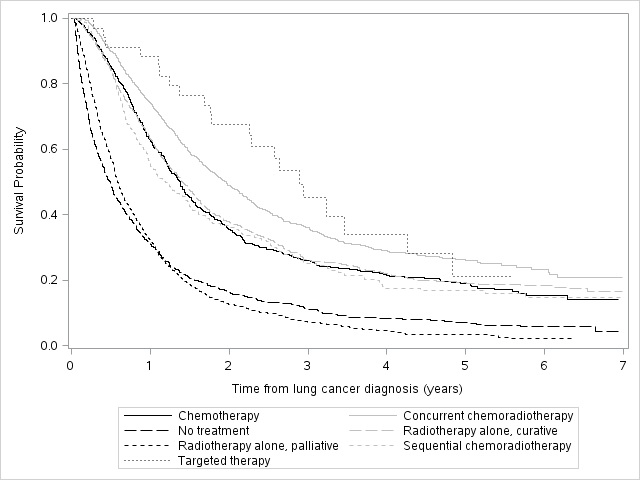
Although cCRT is generally considered standard of care for stage III unresectable NSCLC, patients in Ontario receive various treatment approaches. Survival outcomes vary widely.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.15-28 - Real World Treatment Patterns and Survival of Stage IV Non-Small Cell Lung Cancer (NSCLC) in Ontario, Canada. (ID 13923)
16:45 - 18:00 | Author(s): William Kenneth Evans
- Abstract
Background
The majority of NSCLC patients are diagnosed with stage IV disease. With the development of targeted therapies for advanced NSCLC, it has become important to understand which patients are being treated with systemic therapies and to what benefit.
a9ded1e5ce5d75814730bb4caaf49419 Method
We conducted a longitudinal, population-level study to determine the treatment patterns and survival in patients with stage IV NSCLC in Ontario, Canada between April 1, 2010 and March 31, 2015 from the Ontario Cancer Registry (OCR). Individuals were further identified as having non-squamous disease, and those who received an EGFR-TKI (afatinib, erlotinib, gefitinib) were assumed to be EGFR mutation-positive (EGFR+). Survival was calculated from date of diagnosis to death.
4c3880bb027f159e801041b1021e88e8 Result
24,729 individuals were diagnosed with NSCLC. Approximately half (12,159; 49.2%) had stage IV disease, including 10,103 with non-squamous disease, of whom 508 were categorized as EGFR+. The mean age for the stage IV non-squamous and EGFR+ cohorts were 68.7±11.0 years and 69.1±10.4 years, respectively; 49.3% and 60.8% were female, respectively. The most frequent treatments for stage IV non-squamous patients were palliative radiotherapy (RT) (46.7%) and systemic therapy (14.9%). Patients received no treatment in 26.7% of cases. 75.6% of the EGFR+ cohort received gefitinib, with the majority receiving no subsequent treatment (44.6%). Of EGFR+ patients receiving a second-line treatment, 20.1% received palliative RT and 18.7% received chemotherapy. Mean and median survival times (IQR) for the stage IV non-squamous patients were 0.9±0.0 years and 0.4 (0.2-1.0) years, respectively. Substantial variation in survival was noted by treatment (Figure 1). Mean and median survival times (IQR) for the EGFR+ cohort were 1.9±0.1 years and 1.5 (0.9-3.0) years, respectively.
Figure 1. Kaplan-Meier survival curves for stage IV non-squamous NSCLC patients based on treatment
8eea62084ca7e541d918e823422bd82e Conclusion
Relatively few patients with stage IV non-squamous NSCLC receive any systemic therapy. Survival is generally very poor, but best in the subgroup of EGFR+ patients.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.10 - Prevention and Tobacco Control (Not CME Accredited Session) (ID 959)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.10-02 - Variations in Smoking Cessation Activities at Ontario’s Regional Cancer Centres (ID 14104)
16:45 - 18:00 | Presenting Author(s): William Kenneth Evans
- Abstract
Background
Tobacco use is the largest preventable cause of cancer and cancer mortality, with approximately 85% of lung cancers resulting from smoking. In 2012, Cancer Care Ontario (CCO) developed a smoking cessation (SC) initiative for cancer patients across the province’s 14 Regional Cancer Centres (RCCs). The purpose of this analysis was to examine variations in the rates of screening for tobacco use and SC referrals for lung and non-lung cancer patients in Ontario RCCs.
a9ded1e5ce5d75814730bb4caaf49419 Method
A descriptive analysis was conducted on data submitted to CCO from 14 RCCs on their SC activities among new ambulatory cancer patients in 2017. Data were aggregated and cleaned resulting in 64,635 patient records; two SC performance indicators (Tobacco Screening and Accepted a SC Referral) were calculated by RCC, for lung and non-lung cancer patients.
4c3880bb027f159e801041b1021e88e8 Result
In 2017, 67.8% of all new cancer patients in Ontario were screened for tobacco use (70.2% of lung cancer and 67.5% of non-lung patients). Screening rates for all cancer patients ranged from 51% to 96% across RCCs, with similar ranges for lung and non-lung patients. Within the RCCs, the screening rate differed by up to 12% between lung and non-lung cancer patients. Approximately 17% of all new cancer patients (15% of non-lung patients), seen at RCCs, were identified as current or recent smokers (smoked within the past 6 months), but the proportion was higher among patients with lung cancer at 37%. Overall, 21.5% of all smokers accepted a SC referral (23.7% lung cancer and 20.9% non-lung patients). Rates ranged from 9% to 43% for all cancer patients, with similar ranges observed across patient groups. Within the RCCs, differences of up to 17% were observed in SC referral acceptance rates between lung and non-lung cancer patients. For both the screening and accepted a referral metrics, the direction of the differences was inconsistent, with higher rates observed in lung cancer patients at some RCCs and lower rates at others.
8eea62084ca7e541d918e823422bd82e Conclusion
In Ontario, more than twice as many lung cancer patients were smokers compared to non-lung cancer patients. Although aggregate provincial Tobacco Screening and Accepted a SC Referral rates showed little difference between these patient groups, large variations in rates for both metrics were observed in RCCs between lung and non-lung patients. Further research is necessary to understand the underlying factors that might be contributing to these wide differences in screening and referral practice.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 964)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.15-09 - The Impact of Treatment Evolution in NSCLC (iTEN) Model: Survival and Cost of Treating Patients with Advanced NSCLC in 2017 (ID 13477)
16:45 - 18:00 | Author(s): William Kenneth Evans
- Abstract
Background
Background: The life expectancy and healthcare costs of treating advanced NSCLC (aNSCLC) patients are expected to rise as new targeted and immuno-oncology (IO) therapies are approved for clinical practice. Here, we have used the iTEN model to estimate the cost of managing aNSCLC patients in Canada in 2017.
a9ded1e5ce5d75814730bb4caaf49419 Method
Methods: The iTEN model development and validation are presented in an accompanying abstract (“The iTEN model: Development and Validation”). A treatment algorithm for EGFRm, T790m, ALK re-arrangement and PD-L1+ aNSCLC patients in 2017 was generated through a modified Delphi process based on anonymous responses from Canadian clinical experts. The generated treatment algorithm was used to estimate the survival and life-time costs of managing patients. Health resource use and cost estimates included drug acquisition and administration, adverse events, laboratory and radiologic monitoring, physician visits and end of life costs (2018 costs). Cost estimates were based on published literature, Ontario formulary listings, Cancer Care Ontario recommendations and the Ontario Case Costing Initiative. The estimation of survival is described in the companion abstract.
4c3880bb027f159e801041b1021e88e8 Result
Results: Survey responses indicated that first-line therapy is consistent with current guideline recommended practice, but that care beyond the second-line is variable, particularly with respect to IO usage. Modelled life expectancy varied based on the molecular subtype of aNSCLC. Costs over the span of an average aNSCLC patient’s life-time were estimated to be $89,899 (range: $61,134-$194,158). In comparison, the life-time cost of treating a Canadian lung cancer patient in 2007 (ie, prior to the introduction of IOs and ALK TKIs), inflated to 2018 dollars, was an estimated $60,678 (de Oliveira et al., 2016).
8eea62084ca7e541d918e823422bd82e Conclusion
Conclusions: Results suggest that aNSCLC patient survival increases in conjunction with increased expenditure. The iTEN model may be used to assess the impacts of evolving treatment paradigms in aNSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53


