Virtual Library
Start Your Search
Phillip Crosbie
Author of
-
+
MA03 - Lung Cancer Screening - Next Step (ID 896)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Screening and Early Detection
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 206 AC
-
+
MA03.01 - Manchester Lung Cancer Screening: Results of the First Incidence Screening Round (ID 12568)
10:30 - 10:35 | Author(s): Phillip Crosbie
- Abstract
- Presentation
Background
The European position on lung cancer (LC) screening has recommended planning for implementation to commence throughout Europe (1). The Manchester lung cancer screening pilot is one of the first real world implementation projects to take place in Europe and to publish baseline results (2). In this abstract we share, for the first time, the results from the first incidence screening round of the Manchester pilot.
a9ded1e5ce5d75814730bb4caaf49419 Method
The methodology and results of the baseline round of the Manchester screening pilot have been published previously (2). In brief, ever smokers, aged 55-74, from deprived areas of Manchester were invited to a free ‘Lung Health Check’ (LHC) in mobile units located at their local shopping centres. The PLCOm2012 LC risk stratification model was incorporated into the LHC and those at high risk of LC (PLCOm2012 ≥1.51%) were offered immediate LDCT in a co-located mobile scanner. At baseline, 75% of attendees were ranked in the lowest deprivation quintile; 56% were at high risk and 1384 screened with LDCT. 3% had LC diagnosed of which 80% were early stage (I+II) and 90% offered curative treatment.
In this round of screening, all high risk individuals screened at baseline with no subsequent diagnosis of LC (screening or non-screening) were invited back for an annual LDCT scan at the same community locations. Exclusion criteria included death, other malignancies under follow-up and CT thorax within 3-months of due screening date. National and GP specific registries were checked for interval LC diagnosis.
4c3880bb027f159e801041b1021e88e8 Result
A total of 1,194 LDCT scans were performed as part of the first incidence round of screening. Overall 28 (2.3%) individuals received a positive scan result and were referred to the MDT. Of these, 18 (1.5%) individuals were diagnosed with LC of which 78% (n=14/18) were lower stage (I-II) and 89% (n=16/18) offered curative treatment. The false positive rate was 0.8% of the screened population as a whole and 36% of those with a positive scan result. There were no interval LCs diagnosed at one year.
The cumulative LC detection rate over the first 12 months of the programme was 4.3% (n=60/1384) of which 80% (n=51/64) were stage I-II.
8eea62084ca7e541d918e823422bd82e Conclusion
Annual LDCT screening of high risk individuals in this real world lung cancer screening implementation project continues to identify a significant number of early stage lung cancers amenable to curative treatment. No interval lung cancers were diagnosed at one year suggesting the baseline selection criteria for screening was appropriate.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P2.09 - Pathology (Not CME Accredited Session) (ID 958)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.09-06 - Expression of PD-L1 on Routine Non-Small Cell Lung Carcinoma Sections: Comparative Assessment of SP263 (Ventana) and 22C3 (DAKO pharmDx) (ID 13019)
16:45 - 18:00 | Author(s): Phillip Crosbie
- Abstract
Background
The SP263 (Ventana Benchmark) antibody as a predictive immunohistochemical marker for pembrolizumab therapy provides an avenue for local testing. Pathologists without access to the Dako Autostainer Link 48 platform (certified for the Dako 22C3 antibody) have been restricted to referrals at external departments, resulting in an increased turnaround time. Here we report the results of our local verification of SP263.
a9ded1e5ce5d75814730bb4caaf49419 Method
Specimens previously assessed for 22C3 PD-L1 expression at either Royal Liverpool Hospital or Queen Elizabeth Hospital Birmingham were selected from the archives of Wythenshawe Hospital. Cases with less than 100 viable residual tumour cells were excluded. The same tumour block was selected for staining with the Roche SP263 clone and specimens were assessed for tumour proportion score (TPS), immune cell proportion and staining intensity. Assays were reported as disagreeing if a differing TPS changed the therapeutic cut-off ranges.
4c3880bb027f159e801041b1021e88e8 Result
Expression levels of 22C3 and SP263 were compared across 100 cases (43 resections, 26 biopsies, 26 lymph node aspirates, 5 node excisions); 59 adenocarcinomas, 33 squamous carcinomas, 8 not otherwise specified (70 primary, 30 metastatic). The TPS ranges (<1%, 1 - 49%, > 50%) were in agreement for 78 samples. Of the 22 cases with differing ranges, 15 reflected a TPS of <10% and 7 had greater differences e.g. 10% versus 60%. Reasons for discrepancies included faint membranous staining on a few of the 22C3 sections (not apparent on SP263), scoring of carcinoma in situ, possible scoring of cells at a deeper block level, and variation in interpretation by the scoring pathologists. The overall Pearson correlation coefficient (r) was 0.9025, p < 0.00001.
Table 1 Comparison of PD-L1 Ventana SP263 and Dako 22C3 tumour proportion scores SAMPLE DIAGNOSIS SP263 >/= 1% >/= 50% Liverpool
22C3
Birmingham
22C3
Discrepancy Pleural biopsy
Adenocarcinoma
1
No
No
1
N/A
No
Pleural biopsy
Adenocarcinoma
10
No
No
60
N/A
Yes
EBUS node
Metastatic adenocarcinoma
0
No
No
0
N/A
No
EBUS node
Metastatic adenocarcinoma
0
No
No
1
N/A
Yes
Resection
Adenocarcinoma
40
Yes
No
10
N/A
No
EBUS node
Metastatic squamous carcinoma
2
No
No
10
N/A
No
Resection
Adenocarcinoma
5
Yes
No
<1
N/A
Yes
EBUS node
Metastatic adenocarcinoma
0
No
No
0
N/A
No
Bronchial biopsy
Squamous carcinoma
10
Yes
No
70
N/A
Yes
Bronchial biopsy
Squamous carcinoma
0
No
No
0
N/A
No
EBUS node
Metastatic squamous carcinoma
<1
No
No
<1
N/A
No
EBUS node
Metastatic adenocarcinoma
<1
No
No
0
N/A
No
Bronchial biopsy
Squamous carcinoma
40
Yes
No
40
N/A
No
Resection
Squamous carcinoma
5
Yes
No
10
N/A
No
Lung biopsy
Adenocarcinoma
0
No
No
0
N/A
No
Lymph node biopsy
Metastatic adenocarcinoma
100
Yes
Yes
100
N/A
No
Resection
Adenocarcinoma
<1
No
No
N/A
<1
No
Lung biopsy
Adenocarcinoma
0
No
No
N/A
0
No
Resection
Adenocarcinoma
0
No
No
0
N/A
No
Resection
Adenocarcinoma
10
Yes
No
N/A
5 to 10
No
Resection
Adenocarcinoma
20
Yes
Yes
N/A
20-30
No
Resection
Squamous carcinoma
2 to 4
Yes
No
N/A
3 to 5
No
Bronchial biopsy
Squamous carcinoma
<1
No
No
N/A
<1
No
Bronchial biopsy
Squamous carcinoma
2 to 4
Yes
No
1
N/A
No
Lymph node biopsy
Metastatic adenocarcinoma
100
Yes
Yes
100
N/A
No
Bronchial biopsy
NOS
<1
No
No
N/A
<1
No
Bronchial biopsy
Adenocarcinoma
0
No
No
1
N/A
Yes
Resection
Adenocarcinoma
2 to 4
Yes
No
10
N/A
No
Lymph node biopsy
Metastatic squamous carcinoma
80
Yes
Yes
70
N/A
No
EBUS node
Metastatic adenocarcinoma
80
Yes
Yes
80
N/A
No
Bronchial biopsy
Squamous carcinoma
0
No
No
N/A
<1
No
EBUS node
Metastatic adenocarcinoma
90
Yes
Yes
80
N/A
No
Pleural biopsy
Adenocarcinoma
0
No
No
10
N/A
Yes
Bronchial biopsy
Squamous carcinoma
0
No
No
0
N/A
No
Bronchial biopsy
Squamous carcinoma
5
Yes
No
N/A
0
Yes
EBUS node
Metastatic squamous carcinoma
50
Yes
Yes
50
N/A
No
Bronchial biopsy
Squamous carcinoma
2 to 4
Yes
No
10
N/A
No
EBUS node
Metastatic adenocarcinoma
70
Yes
Yes
N/A
70
No
EBUS node
Metastatic adenocarcinoma
100
Yes
Yes
100
N/A
No
Bronchial biopsy
Squamous carcinoma
1
Yes
No
20
N/A
No
Resection
Adenocarcinoma
5
Yes
No
0
N/A
Yes
Resection
Adenocarcinoma
0
No
No
0
N/A
No
EBUS node
Adenocarcinoma
<1
No
No
0
N/A
No
Resection
Adenocarcinoma
5
Yes
No
10
N/A
No
EBUS node
Sarcomatoid carcinoma
0
No
No
0
N/A
No
Resection
Adenocarcinoma
0
No
No
N/A
<1
No
Lymph node biopsy
Metastatic squamous carcinoma
0
No
No
N/A
<1
No
Resection
Adenocarcinoma
60
Yes
Yes
N/A
60-70
No
Bronchial biopsy
Squamous carcinoma
0
No
No
0
N/A
No
Pleural biopsy
Adenocarcinoma
2 to 4
Yes
No
10
N/A
No
Lung biopsy
Squamous carcinoma
0
No
No
5
N/A
Yes
EBUS node
Metastatic adenocarcinoma
5
Yes
No
30
N/A
No
Resection
Pleomorphic carcinoma
70
Yes
Yes
80
N/A
No
Resection
Squamous carcinoma
0
No
No
1
N/A
Yes
Bronchial biopsy
Squamous carcinoma
10
Yes
No
20
N/A
No
Resection
Adenocarcinoma
50
Yes
Yes
N/A
10 to 20
Yes
Resection
Adenocarcinoma
0
No
No
N/A
<1
No
Resection
Pleomorphic carcinoma
1
Yes
No
20
N/A
No
Resection
Adenocarcinoma
0
No
No
0
N/A
No
Resection
Adenocarcinoma
10
Yes
No
N/A
2 to 3
No
EBUS node
Metastatic adenocarcinoma
100
Yes
Yes
100
N/A
No
Resection
Adenocarcinoma
5
Yes
No
N/A
<1
Yes
EBUS node
Metastatic adenocarcinoma
100
Yes
Yes
100
N/A
No
EBUS node
Metastatic squamous carcinoma
50
Yes
Yes
N/A
80
No
Resection
Adenocarcinoma
90
Yes
Yes
95
N/A
No
Resection
Adenocarcinoma
<1
No
No
N/A
<1
No
Resection
Adenocarcinoma
0
No
No
1
0
Yes
Resection
Adenocarcinoma
<1
No
No
5
N/A
Yes
Resection
Adenocarcinoma
20
Yes
No
20
N/A
No
Bronchial biopsy
NOS
50
Yes
Yes
60
N/A
No
Resection
Adenocarcinoma
2 to 4
Yes
No
N/A
5 to 10
No
Resection
Adenocarcinoma
0
No
No
10
N/A
Yes
Soft tissue
NOS
1
Yes
No
20
N/A
No
Bronchial biopsy
Sarcomatoid carcinoma
<1
No
No
N/A
<1
No
EBUS node
Metastatic adenocarcinoma
70
Yes
Yes
N/A
60 to 70
No
Pleural biopsy
Adenocarcinoma
80
Yes
Yes
N/A
70
No
Resection
Squamous carcinoma
<1
No
No
1
0
Yes
Resection
Adenocarcinoma
50
Yes
Yes
N/A
5 to 10
Yes
Resection
Adenocarcinoma
10
Yes
No
50
N/A
Yes
Resection
Adenocarcinoma
0
No
No
0
N/A
No
Resection
Squamous carcinoma
1
Yes
No
N/A
<1
Yes
Resection
Squamous carcinoma
100
Yes
Yes
100
N/A
No
Resection
Squamous carcinoma
5
Yes
No
10
No
Resection
Squamous carcinoma
70
Yes
Yes
N/A
70 to 80
No
Resection
Squamous carcinoma
90
Yes
Yes
N/A
10 to 20
Yes
EBUS node
Squamous carcinoma
<1
No
No
N/A
<1
No
EBUS node
Adenocarcinoma
0
No
No
N/A
0
No
EBUS node
Metastatic adenocarcinoma
70
Yes
Yes
N/A
80
No
EBUS node
Metastatic squamous carcinoma
50
Yes
No
N/A
70
No
Resection
Adenocarcinoma
<1
No
No
N/A
<1
No
EBUS node
Metastatic adenocarcinoma
40
Yes
No
30
N/A
No
Resection
Squamous carcinoma
90
Yes
Yes
N/A
100
No
Resection
Adenocarcinoma
40
Yes
No
40
N/A
No
EBUS node
Metastatic adenocarcinoma
80
Yes
Yes
N/A
70 to 80
No
EBUS node
Metastatic squamous carcinoma
1
Yes
No
N/A
<1
Yes
Bronchial biopsy
Squamous carcinoma
1
Yes
No
1
N/A
No
EBUS node
Metastatic adenocarcinoma
5
Yes
No
60
N/A
Yes
Primary EBUS
Pleomorphic carcinoma
100
Yes
Yes
90
N/A
No
Resection
Adenocarcinoma
90
Yes
Yes
90
N/A
No
Lymph node biopsy
Adenocarcinoma
<1
No
No
<1
N/A
No
8eea62084ca7e541d918e823422bd82e Conclusion
Tumour expression profiles of PD-L1 are similar for the 22C3 and SP263 antibodies, with a rate of variation similar to previous reports. Cases that are discrepant may reflect differences in pathologist interpretation rather than the assay.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.11 - Screening and Early Detection (Not CME Accredited Session) (ID 960)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.11-03 - Cardiovascular Risk Prediction in a Community-Based Lung Cancer Screening Programme (ID 12567)
16:45 - 18:00 | Author(s): Phillip Crosbie
- Abstract
Background
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in populations eligible for lung cancer screening. The aim of this study was to determine whether a brief CV risk assessment, delivered as part of a community-based lung cancer screening programme, was effective in identifying individuals at high risk who might benefit from primary prevention.
a9ded1e5ce5d75814730bb4caaf49419 Method
The Manchester Lung Screening Pilot consisted of annual low dose CT (LDCT) over 2 screening rounds, targeted at individuals in deprived areas at high risk of lung cancer (age 55-74 and 6-year risk ≥1.51%, using PLCOM2012 risk model). All participants of the second screening round were eligible to take part in the study. Ten-year CV risk was estimated using QRISK2 in participants without CVD and compared to age (±5 years) and sex matched Health Survey for England (HSE) controls; high risk was defined as QRISK2 score ≥10%. Coronary artery calcification (CAC) was assessed on LDCT scans and compared to QRISK2 score.
4c3880bb027f159e801041b1021e88e8 Result
Seventy-seven percent (n=920/1,194) of screening attendees were included in the analysis; mean age 65.6±5.4 and 50.4% female. QRISK2 and lung cancer risk (PLCOM2012) scores were correlated (r=0.26, p<0.001). Median QRISK2 score was 21.1% (IQR 14.9-29.6) in those without established CVD (77.6%, n=714/920), double that of HSE controls (10.3%, IQR 6.6-16.2; n=714) (p<0.001). QRISK2 score was significantly higher in those with CAC (p<0.001). Screening attendees were 10-fold more likely to be classified high risk (OR 10.2 [95% CI 7.3-14.0]). One third (33.7%, n=310/920) of all study participants were high risk but not receiving statin therapy for primary CVD prevention.
8eea62084ca7e541d918e823422bd82e Conclusion
Opportunistic CVD risk assessment within a lung cancer screening programme is feasible and is likely to identify a very large number of individuals suitable for primary prevention.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.11 - Screening and Early Detection (Not CME Accredited Session) (ID 977)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.11-24 - “To Know or Not to Know ...?” Push and Pull in Ever Smokers Lung Screening Uptake Decision Making Intentions (ID 11782)
12:00 - 13:30 | Author(s): Phillip Crosbie
- Abstract
Background
Despite introduction in America and calls for European implementation, lung screening isn't currently endorsed as a UK programme. Whether smokers want to be screened has been raised as an issue. This study explored uptake decision-making with ever-smokers, aged 50-80 as part of the UK’s first community based one-stop lung screening pilot service.
a9ded1e5ce5d75814730bb4caaf49419 Method
Thirty-three participants (22 ex-smokers; 11 smokers) men and women, aged 50-80 were recruited purposively from community settings and health facilities in Manchester, England. The setting is a city with significant deprivation and high lung cancer incidence. Six semi-structured focus groups were held with separate groups for current and former smokers to facilitate freer expression and comparison but mixed by gender, age, ethnicity and deprivation. Discussions followed semi-structured topic guides were audio-recorded, transcribed verbatim and coded using NVIVO software. Inductive thematic analysis was used to analyse data and identify key themes.
4c3880bb027f159e801041b1021e88e8 Result
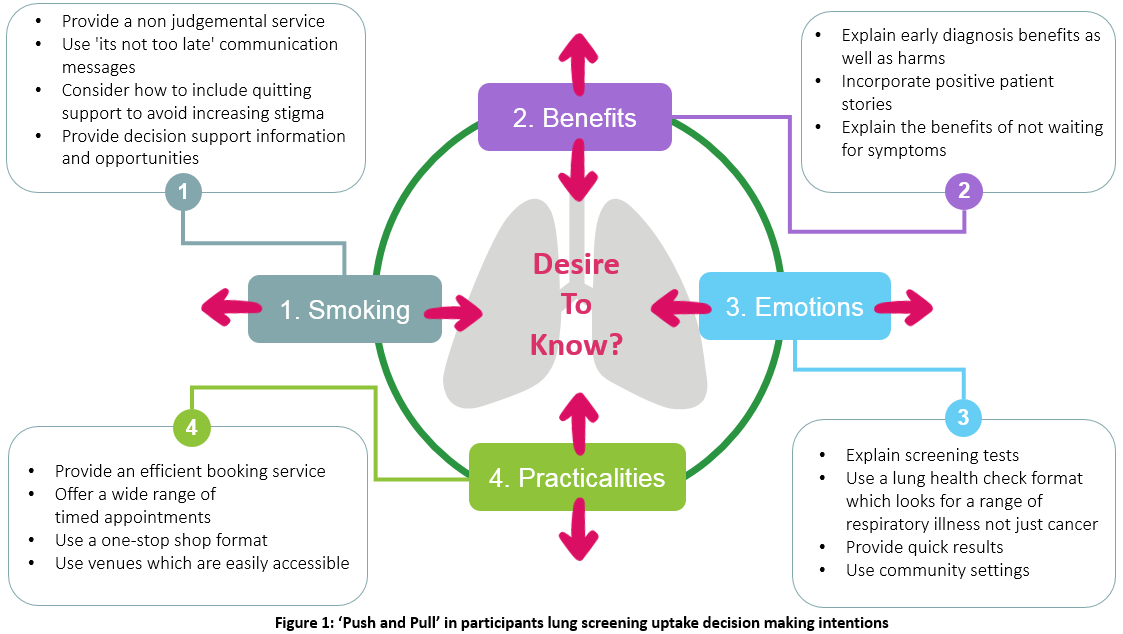
Lung screening was widely acceptable to participants. It was seen as offering reassurance about lung health or opportunity for early detection and treatment. However, being positive ‘in principle’ didn’t always translate into uptake intention. Factors that impacted participants' desire to know about their lung health included: views about screening benefits; emotions such as worry about a diagnosis and screening tests; practicalities such as service accessibility; and smoking related factors included views about individual smoking risk and smoking stigma.
8eea62084ca7e541d918e823422bd82e Conclusion
Indications were that current smokers faced higher uptake barriers than ex-smokers. The uptake factors identified appeared to motivate some participants to be screened but act as a barrier for others. This factorial 'push and pull' effect is important as it indicates where action can be taken to help reduce participation barriers to lung screening. This is shown in Figure One.
6f8b794f3246b0c1e1780bb4d4d5dc53