Virtual Library
Start Your Search
George R. Simon
Author of
-
+
OA01 - Improving Outcomes in Locoregional NSCLC I (ID 892)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Treatment of Locoregional Disease - NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 107
-
+
OA01.06 - DETERRED: Phase II Trial Combining Atezolizumab Concurrently with Chemoradiation Therapy in Locally Advanced Non-Small Cell Lung Cancer (ID 12842)
11:25 - 11:35 | Author(s): George R. Simon
- Abstract
- Presentation
Background
While consolidation immunotherapy after chemoradiation (CRT) is the current standard of care for locally advanced NSCLC (LA-NSCLC), the effectiveness of immunotherapies may be enhanced when combined concurrently with CRT. We report on the safety and preliminary efficacy of combining PD-L1 blockade using atezolizumab (atezo) and concurrent CRT followed by consolidation full dose carboplatin/paclitaxel (CP) with atezo and maintenance atezo up to 1 year for LA-NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
This is a single institution phase II study in LA-NSCLC assessing the safety and feasibility of adding atezo to CRT in two parts: I) sequentially (N=10) with CP after completing CRT, or II) concurrently (N=30) with CRT followed by consolidation atezo with CP. Atezo was given at 1200 mg IV Q3 weeks for up to one year from the first dose. Radiation dose at 60-66 Gy in 30-33 fractions was combined with weekly low dose CP, followed by 2 cycles of full dose CP. Severe adverse events (AEs) ≥ grade 3 are defined within 15 weeks of start of therapy or any immune-related AEs during atezo treatment. Evaluable patients (pts) have received at least one dose of atezo.
4c3880bb027f159e801041b1021e88e8 Result
From February 2016 to April 2018, we accrued 40 evaluable pts. For part 1, any grade 3+ AEs was seen in 6 pts (60%), with most common being pneumonia (2 of 10, 20%). Three grade 3+ AEs (30%) were attributed to atezo, including dyspnea, arthralgia and a grade 5 TE fistula. Grade 2 radiation pneumonitis (RP) was seen in 3 pts. Four progressed with disease during atezo maintenance and have died, ranging from 0.93 to 1.86 years. Four pts completed atezo and are in follow up without recurrence. For part 2, 17 of 30 pts had any grade 3+ AEs (57%), with pneumonia being the most common (6 of 30, 20%). Three (10%) were attributed to atezo (dyspnea, fatigue and heart failure). RP was seen in 3 pts, with 2 grade 2 and 1 grade 3, which led to atezo discontinuation. So far, 4 pts have progressed and 4 have died, 2 due to disease and 2 due to treatment (neutropenic sepsis and gastric hemorrhage). All others have completed CRT and are on maintenance atezo, ranging from 5 to 19 doses. Updated efficacy results will be presented.
8eea62084ca7e541d918e823422bd82e Conclusion
Concurrent atezo with CRT followed by consolidation and maintenance atezo appears safe without increased toxicities compared to CRT alone followed by consolidation and maintenance atezo.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA02 - Novel Therapies in ROS1, HER2 and EGFR (ID 893)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 105
-
+
OA02.06 - A Phase II Trial of Poziotinib in EGFR and HER2 exon 20 Mutant Non-Small Cell Lung Cancer (NSCLC) (ID 14277)
11:25 - 11:35 | Author(s): George R. Simon
- Abstract
- Presentation
Background
Insertions/mutations in exon 20 of EGFR or HER2 occur in ~3% of all lung adenocarcinomas. These alterations are characterized by primary resistance to tyrosine kinase inhibitors (TKIs) with response rates of <12%. We previously showed that exon 20 insertions restrict the size of drug-binding pocket, limiting binding of most available TKIs. However, poziotinib can potentially circumvent these steric changes due to its smaller, flexible structure and is a potent inhibitor of EGFR and HER2 exon 20 mutants (Robichaux et al. Nat Med, 2018). Herein, we report the results of an investigator-initiated study of poziotinib in EGFR and HER2 exon 20 mutant NSCLC (NCT03066206).
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients ≥18yrs with metastatic NSCLC bearing mutations/insertions in EGFR or HER2 exon 20 (except EGFR T790M) were eligible. Unlimited prior systemic and targeted therapies were permitted. Poziotinib 16mg PO daily was administered until progression, death, or withdrawal. The primary endpoint was objective response rate (ORR) based on RECIST v1.1. Response was evaluated every eight weeks. A Bayesian design was used with a plan to enroll patients in cohorts of 10 and to terminate the study if ORR was ≤20%. Secondary endpoints included DCR, PFS, OS and safety
4c3880bb027f159e801041b1021e88e8 Result
As of May 3, 2018, the planned EGFR cohort of 50 patients was fully enrolled, and 40 patients were evaluated for response. 65.1% of patients had received at least two prior lines of therapy for metastatic disease. 60% of patients had ≥grade 3 adverse events; most common were skin-rash (27.5%) and diarrhea (12.5%). 45.0% of patients required dose reduction to 12mg, while 17.5% required dose reduction to 8mg. One patient stopped treatment due to grade 3 skin rash. ORR at eight weeks was 58% (95%-CI 40.9-73.0) and the DCR was 90% (95%-CI 76.3-97.2). Among 23 patients who achieved partial response, 15 responses were confirmed with subsequent scans, five responses were unconfirmed, and three patients are pending confirmation. Responses were observed in 8/13 (62%) patients that were previously treated with TKI. Median PFS was 5.6mo (95%-CI 5.06-NA). Furthermore, 13 patients were enrolled in HER2 cohort. Toxicities were similar to EGFR cohort except one case of grade 5 pneumonitis, assessed to be possibly drug related. Twelve patients were evaluated for response with ORR of 50% (95% CI 21.1-78.9) at eight weeks and DCR of 83%.
8eea62084ca7e541d918e823422bd82e Conclusion
In heavily pre-treated population with EGFR and HER2 exon 20 mutant NSCLC, poziotinib demonstrated encouraging antitumor activity in both TKI-naive and -refractory patients, and manageable toxicity profile.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-37 - Clinical Evaluation of Plasma-Based (cfDNA) Genomic Profiling in Over 1,000 Patients with Advanced Non-Small Cell Lung Cancer (ID 14332)
16:45 - 18:00 | Author(s): George R. Simon
- Abstract
Background
Tumor genomic information from a simple blood collection revealing actionable mutation can improve clinical outcome without the need for an invasive tissue biopsy. We report on the clinical utility of a cell-free DNA (cfDNA) next generation sequencing (NGS) blood test in our patients with non-small cell lung cancers (NSCLC) and the outcome of treatments with targeted therapies based on the reported mutations.
a9ded1e5ce5d75814730bb4caaf49419 Method
From May 2015 to February 2017, 1078 blood samples from 1011 consecutive patients with a diagnosis of NSCLC were collected and analyzed using next-generation sequencing of cfDNA with a panel of up to 70 cancer-related genes at a CLIA-certified lab (Guardant360, Guardant Health, Redwood City, CA) with reported sensitivity of 0.02% mutant allele fraction with high specificity (> 99.9999%) (CCR 2018 (17):3831). Patients in this retrospective study received targeted therapy as indicated by cfDNA molecular profiling. Tumor response was evaluated by RECIST V1.1 and standard clinical evaluation.
4c3880bb027f159e801041b1021e88e8 Result
From 1011 patients, 1078 cfDNA tests sent (additional follow-up tests: 1 in 64 patients and 2 in 3 patients). In 223/1011 (22%) patients had cfDNA report with at least 1 targetable mutations; with 48/223 (22%) patients meeting criteria for this retrospective review. Study population were 31 female:17 male, median age of 63 years (ranged:31-94). The rationale for the blood test included: insufficient tissue or not available (32%), addition to tissue molecular analysis (17%), alternative to tissue biopsy(10%), on-going treatment evaluation/resistance (41%). Mutations included:EGFR T790M (15), EGFR exon 19del (12), EGFR L858R (9), EGFR exon 20 insertion (4), EGFR others (1), ALK gene fusions (5) and MET exon 14 skipping (2). The median line of therapy was 2(ranged:1-7) with 28 patients receiving TKI as 1st line of therapy based on cfDNA mutations. With targeted treatments based on ctDNA results, the responses (RECIST V1.1) were: CR(3), PR(26), SD(14) and PD(4); median PFS was 8.5 months (ranged:1-26mos) for the overall population with 4 patients still receiving targeted therapy. Median PFS was 9.5 months (ranged:1-20 months) for those receiving TKI as 1st line.
8eea62084ca7e541d918e823422bd82e Conclusion
This is the largest analysis of response rates with cfDNA directed therapy in advanced NSCLC and demonstrates positive clinical outcomes in patients treated with targeted therapy based on plasma identified biomarkers.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-87 - Profiling the Symptom Burden of Patients with Metastatic NSCLC Receiving Either Chemotherapy or Targeted Therapy: Real-World Data (ID 13348)
16:45 - 18:00 | Author(s): George R. Simon
- Abstract
Background
An understanding of the patient experience is lacking for newly developed cancer treatments, such as targeted therapies. We profiled the patient-reported outcome (PRO)-measured symptom burden experienced by patients with metastatic non-small cell lung cancer (mNSCLC) during 6 months of conventional chemotherapy or targeted therapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
During 2017, patients with mNSCLC at a single institution were recruited and completed the MD Anderson Symptom Inventory lung cancer module (MDASI-LC) at clinic visits. The MDASI-LC assesses the severity of 13 core and 3 lung-cancer-specific symptoms and 6 interference items on 0‒10 scales (0=no symptom or interference, 10=worst imaginable symptom or complete interference). Descriptive statistics for MDASI-LC scores over 6 months of treatment were summarized. Symptom trajectories for the chemotherapy patients versus the targeted-therapy patients were compared via linear mixed-effects models.
4c3880bb027f159e801041b1021e88e8 Result
Of 65 patients receiving chemotherapy and 27 receiving targeted therapy, the targeted-therapy group had more women (74% vs. 49%, P=0.029) and younger patients (57.6±12.2 vs. 64.2±9.9 years, P=0.012). Before treatment, both groups reported similar symptom burden, although sadness was worse in the targeted-therapy group (2.4±1.6 vs. 0.8±1.5, P=0.021). During the first 60 days of treatment, patients receiving chemotherapy reported significant increase in pain (estimate (est)=0.03, P=0.037) and interference with walking (est=0.04, P=0.025). Compared with those receiving chemotherapy, patients receiving targeted therapy experienced significantly less severe pain (est=‒1.17, P=0.024), fatigue (est=‒1.16, P=0.019), and shortness of breath (est=‒1.23, P=0.028) and less interference with walking (est=‒1.23, P=0.042) (figure 1). More severe dry mouth was reported by patients undergoing targeted therapy (est=1.17, P=0.027).
8eea62084ca7e541d918e823422bd82e Conclusion
This real-world data demonstrates that, compared with conventional chemotherapy, targeted therapy correlates with less impairment of physiological condition and functioning in patients with mNSCLC. Additional follow up will confirm and expand these findings about the patient experience relative to treatment response.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.04 - Immunooncology (Not CME Accredited Session) (ID 953)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.04-09 - Driver Mutations are Associated with Distinct Patterns of Response to Immune Checkpoint Blockade in Non-Small Cell Lung Cancer (ID 13362)
16:45 - 18:00 | Author(s): George R. Simon
- Abstract
Background
Immune checkpoint blockade (IO) has demonstrated durable clinical benefit in metastatic non-small cell lung cancer (NSCLC). Tumors with driver mutations such as EGFR exon 19 and 21 mutations and ALK translocation tend to have low response rates to IO. However, IO response in NSCLC patients with rare driver mutations, such as EGFR exon 20 (~2%), HER-2 (~2%) and BRAF (~3%), representing approximately 7% of lung adenocarcinomas, has been poorly addressed.
a9ded1e5ce5d75814730bb4caaf49419 Method
We queried GEMINI (MD Anderson Lung Cancer Moon Shot funded database for prospective collection of clinical information on NSCLC) for patients with mutations in EGFR exon 19, 20, 21, HER-2 and BRAF treated with PD-1/PD-L1 checkpoint inhibitors. We assessed progression-free survival (PFS), overall response rate (ORR) and overall survival (OS) in each molecular group.
4c3880bb027f159e801041b1021e88e8 Result
Between 2014-2018, 108 patients with classic EGFR mutations (exon 19 del + exon 21 L858R, n=37), EGFR exon 20 mutations (n=36; no T790M included), HER-2 mutations (n=22) and BRAF mutations (n=13; V600E: 3pts; non-V600E: 10pts) had been treated with PD-1/PD-L1 inhibitors. EGFR exon 20 mutants and BRAF mutants demonstrated significantly higher PFS (EGFR exon 20: HR 0.4, p<0.001; BRAF: HR 0.2, p<0.001), higher disease control rate at 6 and 12 months as well as higher ORR when compared to classic EGFR mutants (Table). These differences remained significant in multivariate analysis after adjusting for age, smoking, PD-L1 status, radiation prior to treatment initiation, treatment with concurrent agents and prior treatment with TKIs. HER-2 mutants had similar PFS compared to EGFR classic mutants (HR 0.8, p=0.35) (Table).
8eea62084ca7e541d918e823422bd82e Conclusion
EGFR exon 20 and BRAF mutations are associated with superior outcome from PD-1/PD-L1 checkpoint inhibitors compared to classic EGFR and HER-2 mutations. Further studies on co-mutational status and tumor mutation burden in these molecularly-defined groups are ongoing to address potential underlying mechanisms associated with these findings.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-109 - Real-World Patient-Reported Outcome Assessment of Patients with Metastatic Non-Small Cell Lung Cancer (ID 12213)
12:00 - 13:30 | Author(s): George R. Simon
- Abstract
Background
Patient-Reported Outcomes (PROs) provide information on patient treatment experience. We have established a real-world Advanced Non-Small Cell Lung Holistic Registry (ANCHoR) to understand how the advent of immunotherapy impacts treatment choice, clinical outcomes, and PROs of metastatic non-small cell lung cancer (mNSCLC). The aim of this analysis is to report early results of baseline symptom status and quality of life among mNSCLC patients using the MD Anderson Symptom Inventory lung cancer module (MDASI-LC) and EuroQol-5D 5-level version (EQ-5D-5L).
a9ded1e5ce5d75814730bb4caaf49419 Method
During 2017, patients with mNSCLC at a single institution were enrolled in ANCHoR and completed the PRO questionnaires at clinic visits. MDASI-LC consists of thirteen core and three lung cancer-specific symptom severity questions, and six interference items rated on 0-10 scales (0 = no symptom or interference, 10 = worst imaginable symptom or complete interference). EQ-5D-5L captures five health state dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression rated on a five-level scale (1= no problems, 5= extreme problems). A single visual analogue scale (VAS) on EQ-5D-5L records patient self-rated health between ”best imaginable” (100) and “worst imaginable” (0) health state. Descriptive statistics for PRO scores at baseline are summarized.
4c3880bb027f159e801041b1021e88e8 Result
Forty-two patients completed baseline PROs before the start of therapy. Mean patient age was 63 years and 45% were males. For MDASI-LC, the mean scores for the core symptom, lung cancer-specific symptom, and interference subscales at baseline were 2.2 (standard deviation [SD] = 2.80), 2.1 (SD = 2.80), and 2.8 (SD = 3.10), respectively. Fatigue was the most severe symptom reported at baseline (mean = 4.1, SD = 3.01), followed by shortness of breath (mean = 3.2, SD = 2.81) and pain (mean = 3.19, SD = 3.00). The highest percentages of patients reporting moderate to severe symptom levels (score of ≥5) were 38% for fatigue, 33% for pain, 31% for drowsiness, 29% for shortness of breath and disturbed sleep, and 26% coughing. For EQ-5D-5L, 91% of patient reported problems with self-care, 81% with mobility, 48% with usual activity and anxiety, and 33% with pain. Mean EQ-5D VAS was 73.9 (SD = 18.2).
8eea62084ca7e541d918e823422bd82e Conclusion
Prior to the start of treatment, fatigue, pain, drowsiness, disturbed sleep, and coughing were the most common symptoms with fatigue, shortness of breath, and pain being the most severe. Additional follow up will confirm and expand these findings and will also allow us to examine change in PROs after first-line treatment is administered.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P3.01-91 - Computing the Impact of Immunotherapy on the Non-Small Cell Lung Cancer (NSCLC) Therapeutic Landscape (ID 12209)
12:00 - 13:30 | Presenting Author(s): George R. Simon
- Abstract
Background
The Advanced Non-Small Lung Holistic Registry (ANCHoR) is established to examine the real-world impact of immunotherapy on choice of treatment, clinical outcomes, and patient reported outcomes of patients with Stage IV NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
Stage IV NSCLC patients diagnosed or initiating treatment at MD Anderson from January 1, 2017 are enrolled in the ongoing ANCHoR study. Their demographic, clinicopathological, molecular, and treatment data were populated in a prospective database. Treatment patterns by line and PD-L1 status were summarized in this interim analysis.
4c3880bb027f159e801041b1021e88e8 Result
At the time of data cut off (Dec 31, 2017) 182 patients were enrolled in the registry, of which 150 were tested for PD-L1. Number of patients initiating first-, second-, and third-line treatment were 163, 42 and 7, respectively. Of the 30 patients not tested for PD-L1, 10 did not have enough tissue and 8 had actionable mutations.
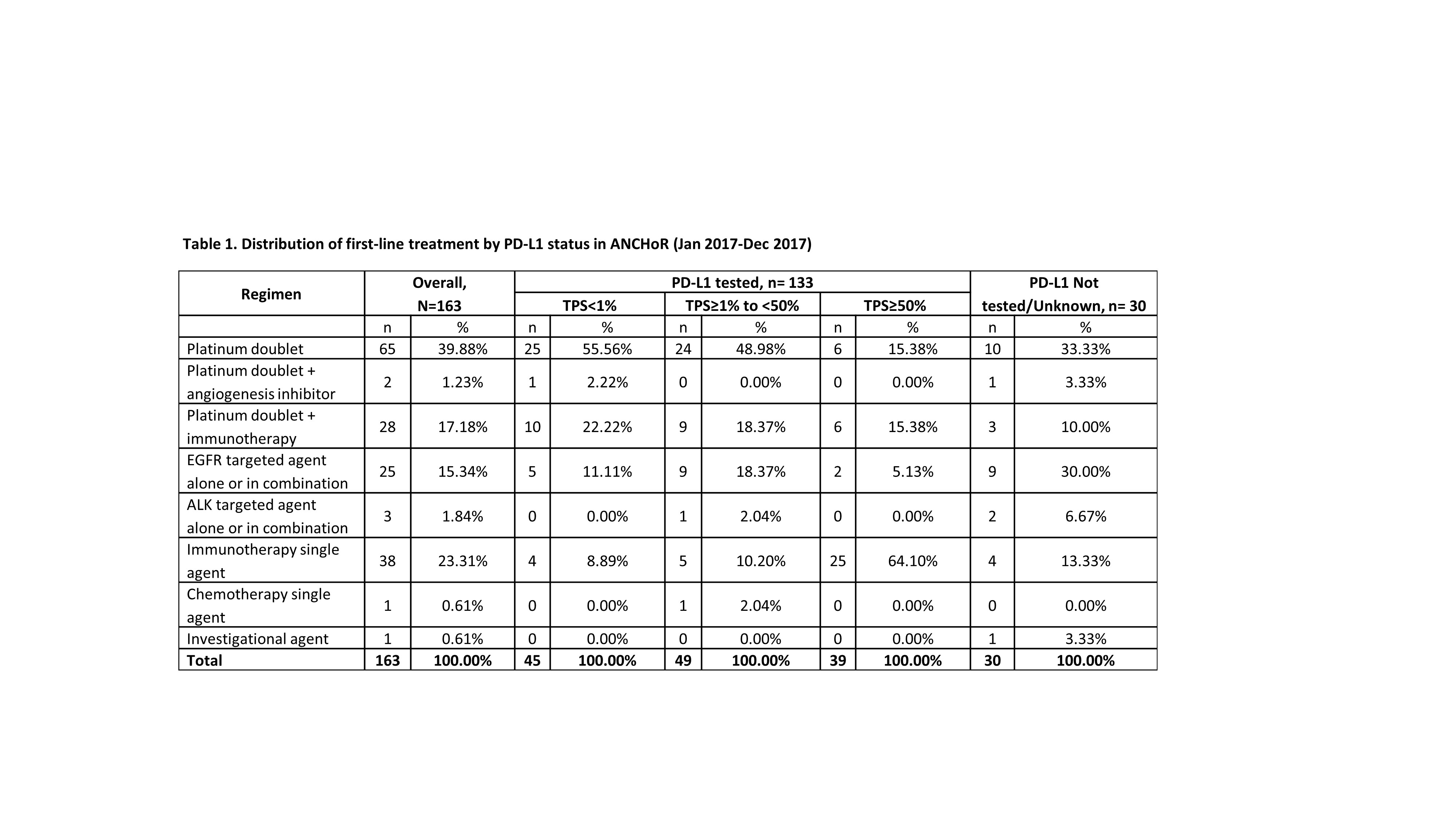
8eea62084ca7e541d918e823422bd82e Conclusion
The emergence of immunotherapy has had a dramatic impact on the first-line treatment of patient with advanced NSCLC. As of December, 2017 up to 41% of patient received immunotherapy either singly (23%) or in combination with chemotherapy. Only 40% of the patients now receive chemotherapy alone. There has been dramatic decrease in the use of chemotherapy with an anti-angiogenesis agent (1.23%). In our dataset 16% of the patients were eligible for targeted therapy as initial treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 981)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.15-29 - Defining the Symptom Burden of Non-Small Cell Lung Cancer (ID 12361)
12:00 - 13:30 | Author(s): George R. Simon
- Abstract
Background
Symptom burden is disease and treatment symptom severity and its impact on daily functioning. Symptom monitoring has demonstrated improved cancer patient outcomes, including quality of life, resource utilization, ability to continue treatment, and survival. The use of disease-specific patient-reported outcomes (PRO) measures facilitates individualized symptom monitoring and management. The purpose of this study was to describe symptom experience from the patient perspective and identify key symptoms for a PRO measure of non-small cell lung cancer (NSCLC) symptom burden.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with NSCLC described their symptom experience in single qualitative interviews. Content analysis was used to define the content for a PRO measure of NSCLC symptom burden.
4c3880bb027f159e801041b1021e88e8 Result
Mean age of the 40 patients interviewed was 66.1 years (standard deviation = 10.9); 60.0% were male, 77.5% were white, and 56.4% had stage IV disease. Content analysis found a total of 32 symptoms, 6 reported by ≥ 20% of participants (see Table 1). Symptoms varied based on treatment modality (chemotherapy versus radiation therapy), but not stage of disease. Numbness or tingling and sore mouth were described only by patients who had received chemotherapy. Patients volunteered ways in which symptoms impacted daily activities and relationships.
Table 1. Patient quotes from qualitative interviews describing the 6 most common symptoms (reported by ≥ 20% of participants) Symptom Participant Quote Shortness of breath “The heaviness, it’s like a—wow, I don’t know how to explain it—like a rock and hard to breathe sometimes, just shortness of breath. Of course, the more I try to walk, or whatever, I’m more short of breath.”
‒ 67-year-old femaleCough “I had a real bad cough. I think I actually even broke a couple of ribs coughing so much.”
‒ 52-year-old male
Distress “Terrifying. There’s no ways about it. You know, it’s a terrifying experience, especially when it’s dropped in your lap and you have to deal with it. You go through a lot physically and mentally.”
‒ 67-year-old maleFatigue “I'm more tired. I take a lot of naps where I never had been a nap person. Before I had all my energy, and I was doing lots of things, and now I'm wore out. I wake up, and I'm wore out.”
‒ 53-year-old malePain “You keep trying to move it to make it feel better and no matter where you put it, it doesn’t feel any better … most of the time it will bother me after I get out of bed in the morning for a while. And then if I go try to take a nap, I’ll go ahead and take something for pain because I can’t lay there and—I just keep moving it and moving it and nothing helps.”
- 68-year-old maleConstipation “I didn’t have a bowel movement. I had always taken the stool softeners because they told me to do that. And I kept thinking, “Well, it’s going to work. It’s going to work.” Finally, I was in so much pain that I couldn’t stand anymore, so I went to the hospital … and they ended up physically removing, which was horrible.”
‒ 68-year-old female
8eea62084ca7e541d918e823422bd82e Conclusion
Patients with NSCLC experience numerous symptoms related to disease and treatment. Shortness of breath, cough, distress, fatigue, pain, and constipation were commonly reported symptoms, suggesting that clinicians should routinely and proactively monitor the presence and severity of these symptoms in NSCLC clinical care. In patients receiving chemotherapy, attention to specific treatment-related symptoms, including symptoms of neuropathy and sore mouth, is needed. While stage of disease does not produce unique symptoms, the severity of the symptoms may possibly vary by stage of disease. Clinicians should also be aware that symptoms result in interference with daily activities, relationships, life plans, treatment adherence, and mood.
6f8b794f3246b0c1e1780bb4d4d5dc53


