Virtual Library
Start Your Search
Natasha B Leighl
Author of
-
+
MA02 - Improving Outcomes for Patients with Lung Cancer (ID 895)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 201 BD
-
+
MA02.11 - Achieving Value in Cancer Diagnostics: Blood Versus Tissue Molecular Profiling - A Prospective Canadian Study (VALUE) (ID 13611)
11:40 - 11:45 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
Cell-free DNA (cfDNA) next-generation sequencing (NGS) has emerged as an effective molecular profiling technique that is potentially faster and cost-saving in comparison to standard-of-care (SOC) tumour biopsy and tissue-based profiling. In a public payer system, the added value of cfDNA blood-based profiling compared to SOC remains unknown. This study will determine the incremental clinical utility and cost of cfDNA NGS versus SOC genotyping in patients with advanced non-squamous non-small cell lung cancer (NSCLC).
a9ded1e5ce5d75814730bb4caaf49419 Method
This multicentre, non-randomized, longitudinal study will be conducted at 6 sites across Canada (BC, Alberta, Ontario, Quebec). The Guardant360® assay will be used to perform plasma-based cfDNA testing, and includes mutations, rearrangements and copy number variations in 73 known cancer associated genes. Two patient cohorts will be recruited: (1) treatment naïve patients with ≤10 pack year smoking history; and (2) patients with known abnormalities of EGFR, ALK, ROS-1 or BRAF after disease progression on all standard targeted therapies. SOC tissue profiling will be performed for all patients per institutional standards. The study will begin recruiting in May 2018, with estimated completion in 12 months. The primary endpoints are comparison of response rate (RR), progression-free survival (PFS) and time-to-treatment failure (TTF) using cfDNA versus tissue genomic testing. Secondary endpoints include time to treatment initiation, number of actionable genomic abnormalities identified, result turnaround time, potentially avoidable repeat tissue biopsies, costs, patient-reported quality of life (EQ-5D) and willingness-to-pay. Exploratory analyses of treatment outcomes in selected molecular subgroups will also be undertaken, including response to immunotherapy in those with KRAS/STK11 co-mutations. A decision-analytic model will be developed to perform cost-consequence analyses using a cfDNA versus tissue-based approach.
4c3880bb027f159e801041b1021e88e8 Result
A total of 210 patients will be recruited across Canada, (Cohort 1 N=150, Cohort 2 N=60). Based on testing with either blood-based GUARDANT360TM or tissue-based profiling, the costs and benefits of blood-based profiling either at initial diagnosis or upon TKI progression will be determined versus initial or repeat tumour biopsy and tissue-based profiling. Data from patients accrued until 08/2018 will be presented at the meeting.
8eea62084ca7e541d918e823422bd82e Conclusion
This study will determine the added value of cfDNA blood-based genotyping compared to SOC from the perspective of a public payer system (Canada).
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA14 - Survivorship, Socioeconomic and End-of-Life Considerations (ID 915)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment in the Real World - Support, Survivorship, Systems Research
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 10:30 - 12:00, Room 205 BD
-
+
MA14.06 - Predictors of Financial Toxicity, an Under-Recognized Patient-Reported Outcome (ID 13571)
11:05 - 11:10 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
In contemporary cancer care, financial distress has been established as a clinically relevant patient-reported outcome (PRO) associated with worse mortality and quality of life, but remains under-recognized by health care providers. Our goal was to define predictors of patient financial toxicity (FT) in a public healthcare system.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with advanced lung cancer were recruited from outpatient clinics at the Princess Margaret Cancer Centre (Toronto, Canada). FT was measured with the validated Comprehensive Score for Financial Toxicity (COST) instrument, an 11-item survey scored from 0-44 with lower scores reflecting worse financial well-being. Data on patient and treatment characteristics, total out-of-pocket costs (OOP) and extended insurance coverage (EIC) were collected. Associations between variables and COST score were evaluated using multivariable regression analyses.
4c3880bb027f159e801041b1021e88e8 Result
Of 249 patients approached, 200 (80%) participated. Median age of the cohort was 65 years; 44% were male, 36% immigrants, 67% employed or on pension, with median OOP between $1000-5000 CAD. Median COST score was 21 (range 0-44). FT was associated with age, with patients <65 years reporting greater FT than older patients (COST 18 vs. 25; P<0.0001). Employed patients or those receiving pension income reported less FT than unemployed patients (22 vs. 19; P=0.01). Less FT occurred in patients with EIC compared to those without (23 vs. 19; P=0.03). Patients with higher OOP reported more FT (P<0.0001). Patients on clinical trials reported less FT than others (25 vs. 20; P=0.04). In multivariable linear regression, younger age was a predictor of higher FT, when adjusting for income, employment status, OOP and EIC (P<0.0001).
8eea62084ca7e541d918e823422bd82e Conclusion
Age is a predictor of FT in the Canadian (Ontario) public healthcare system, with younger lung cancer patients reporting greater financial distress. This study highlights priority patient populations where FT should be routinely assessed and appropriate resources for support offered.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA18 - Modelling, Decision-Making and Population-Based Outcomes (ID 920)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment in the Real World - Support, Survivorship, Systems Research
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 201 F
-
+
MA18.07 - Awareness of the Harms of Continued Smoking Among Lung Cancer (LC) Survivors (ID 12024)
14:05 - 14:10 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
Continued smoking after a LC diagnosis is associated with poorer cancer outcomes including increased risk of treatment-related side-effects, reduced treatment efficacy and poorer prognosis. Smoking cessation is an integral part of LC survivorship by improving both cancer and non-cancer outcomes. To enhance survivorship education, clinicians should understand patient awareness of the harms of continued smoking.
a9ded1e5ce5d75814730bb4caaf49419 Method
LC survivors from Princess Margaret Cancer Centre, Toronto (2014-2017) were surveyed with respect to self-awareness of the harms of continued smoking on cancer-related outcomes. Univariable and multivariable logistic regression models assessed factors associated with awareness and whether awareness was associated with cessation among current smokers at diagnosis.
4c3880bb027f159e801041b1021e88e8 Result
Of 553 patients, 181 were lifetime never-smokers. Among those smoking during the peri-diagnosis period (n=177), 65% quit after diagnosis. Among all, few patients were aware that smoking negatively impacts treatment-related outcomes [complications from cancer surgery (only 41% aware), radiation side-effects (30%), quality-of-life on chemotherapy (44%) and treatment efficacy (36%)]; half were aware that smoking negatively impacts cancer prognosis (51% aware) and risk of developing second primaries (50%). Compared to ex-smokers/never-smokers at diagnosis, current smokers at diagnosis were less aware of the impact of smoking on radiation side-effects (22% vs 31% aware, P=0.01), prognosis (44% vs 55%, P=0.02) and risk of second primaries (42% vs 55%, P=0.007). Among sociodemographic variables, only those speaking English at home were consistently found more likely unaware that smoking negatively impacts these outcomes (ORs=1.52-2.20, P<0.04). Patients with early stage disease were more likely unaware that smoking negative impacts radiation side-effects (OR=1.60, 95%CI[1.09-2.35], P=0.02); while patients on curative treatment (OR=1.53[1.08-2.17], P=0.02) and those exposed to second-hand smoke (SHS) were more likely unaware that smoking impacts quality-of-life on chemotherapy (OR=1.64[1.05-2.58], P=0.03). Exposure to SHS, treatment intent and stage were not associated with awareness of impact on prognosis or second primaries (P>0.11). Among smokers in the peri-diagnosis period, awareness of the impact of smoking on surgical complications (aOR=2.09 [0.96-4.54], P=0.06), quality-of-life while receiving chemotherapy (aOR=2.60[1.17-5.79], P=0.02) and on treatment efficacy (aOR =2.24[0.97-5.20], P=0.06) were each associated with subsequent quitting, adjusted for marital status, pack-years, self-rated health and SHS exposure.
8eea62084ca7e541d918e823422bd82e Conclusion
Many LC patients are unaware of the harms of continued smoking on cancer outcomes, particularly those smoking at diagnosis. Awareness of some of these outcomes was associated with subsequent tobacco cessation. Patient education on the health benefits of smoking cessation may increase quit rates and improve outcomes for LC patients.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA18.09 - Predictors of Health Utility Scores (HUS) in Advanced EGFR-Mutated NSCLC. (ID 13087)
14:25 - 14:30 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
Advanced NSCLC patients with EGFR mutations (EGFRm) are currently treated with first - to third-generation tyrosine kinase inhibitors (TKIs). In the advanced setting, quality of life is an important goal; we therefore evaluated determinants of HUS in this population.
a9ded1e5ce5d75814730bb4caaf49419 Method
In a prospective, observational study, patients with advanced EGFRm NSCLC completed EQ-5D surveys at outpatient visits generating HUS (range 0-1). Patients were allowed to enrol at any point in their disease course. Baseline clinical characteristics and outcome data were extracted from chart review. Patient imaging was reviewed and health states (stable/progressing) at each encounter recorded. Univariable analyses conducted using ANOVA and multivariable regression analyses with generalized estimating equations identified factors associated with HUS.
4c3880bb027f159e801041b1021e88e8 Result
From November 2014 to July 2017, 782 encounters (follow-up visits) were collected for 244 patients. Median age at first encounter was 64 years (range:29-96); 54% were female and 54% Asian. Median time from diagnosis of stage IV NSCLC to first encounter was 23 months (range:0-67). The median number of HUS collected per patient was 2 (range:1-14). For patients with multiple visits the median time between completed questionnaires was 1.8 months (1-18). 105 patients (43%) presented with or developed brain metastases during the study period. In a univariable analysis, regardless of treatment line, mean HUS (mHUS) on osimertinib was 0.85 (standard deviation (SD):0.15) (n=33 patients; 114 encounters) compared to mHUS=0.80 (SD:0.17) on gefitinib (n=147, 351 encounters); mHUS=0.72 (SD:0.16) on chemotherapy (n=32, 76 encounters); and mHUS=0.79 (SD=0.15) on other TKIs (n=49, 133 encounters); p<0.001. In a multivariable analysis, disease progression (p=0.04) and ECOG performance status >0 (p<0.001) were associated with lower HUS. In contrast, treatment with osimertinib (when compared to a reference group of first-generation TKIs, gefitinib/erlotinib) was associated with improved HUS (p=0.01), while line of therapy and number of metastatic sites of disease were not associated with HUS. In addition, brain metastases had no significant impact on HUS (p=0.33).
8eea62084ca7e541d918e823422bd82e Conclusion
Progressive disease and worse performance status associate with lower HUS in patients with EGFRm NSCLC. Patients treated with osimertinib had the highest HUS when compared with a reference group of first-generation EGFR TKIs regardless of line of therapy. These results may help in the choice of EGFR-TKI, especially in patients with a poor performance status.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA23 - Early Stage Lung Cancer: Present and Future (ID 926)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment of Early Stage/Localized Disease
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 105
-
+
MA23.07 - Defining the Role of Adjuvant Therapy for Early Stage Large Cell Neuroendocrine Lung Cancer (ID 12814)
11:10 - 11:15 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
Large cell neuroendocrine lung cancer (LC-NEC) is a rare, high-grade neuroendocrine tumor. Patterns of adjuvant treatment after surgical resection have not been well defined.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with a pathologic diagnosis of LC-NEC were identified in the National Cancer Database 2004-2014. Patient demographics, tumor and treatment characteristics were examined. Survival differences in patients receiving chemotherapy were evaluated using Kaplan-Meier curves, and multivariate hierarchical Cox models were constructed to evaluate the impact of patient, histologic, tumor, treatment and hospital characteristics on overall survival (OS). A conditional landmark of 90-day postoperative survival was used to address immortal time bias and propensity-matching was used to address imbalance in covariates between groups.
4c3880bb027f159e801041b1021e88e8 Result
1,793 patients were identified with pathologically stage I LC-NEC, of which 482 (26.9%) received adjuvant chemotherapy. Use of adjuvant chemotherapy remained similar across the study period. Patients receiving adjuvant chemo were younger, less comorbid and more likely to have T2 tumors. Significantly longer survival was observed with the receipt of adjuvant chemotherapy (5-year OS 59.2% vs. 45.3%), which persisted after adjustment in multivariable Cox models (HR 0.69, 95%CI0.58 – 0.82, p<0.0001). Adjuvant chemotherapy was associated with longer survival in patients with tumors 2-3cm (60.4% vs. 41.8%; HR 0.64, 95%CI 0.46-0.89, p<0.0001), and T2 tumors (59.8% vs. 42.1%; HR 0.63, 95%CI 0.50-0.81, p<0.0001), but no differences were observed for LC-NEC patients with tumor size <2cm. Adjuvant chest radiotherapy was not associated with improved survival. T-stage specific propensity-matching confirmed these findings, however the association between survival and adjuvant chemotherapy for patients with tumors 2-3cm was no longer significant.
8eea62084ca7e541d918e823422bd82e Conclusion
In this national study of LC-NEC, adjuvant chemotherapy was associated with significantly longer survival in Stage I tumors greater than 2cm. Adjuvant radiation was not associated with survival. A randomized trial of stage T2-4N0 LCNEC is needed to clarify the role of adjuvant chemotherapy in this population.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA25 - Oligometastasis: Defining, Treating, and Evaluating (ID 929)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Oligometastatic NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 13:30 - 15:00, Room 203 BD
-
+
MA25.11 - Clinical and Molecular Predictors of Outcome in Patients with EGFR mutant NSCLC Brain Metastases treated with RT (ID 14529)
14:40 - 14:45 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
Brain metastases(BM) develop in ~45% of patients with EGFR mutant(EGFRm) non-small cell lung cancers(NSCLC). There are limited reports on clinical/molecular factors associated with BM outcomes after radiotherapy in EGFRm NSCLC patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
We identified patients with EGFRm NSCLC who presented with or developed BM and had their lung tumor resected. Clinical, demographic and TP53 status were collected from medical/pathology records. Whole-Exome Sequencing of the primary tumor was performed. Overall survival(OS) and intracranial progression(IP) were defined from start of BM treatment and correlated with clinical/molecular features. IP was defined from the date of BM treatment until any brain failure, either local(previously present BM) or distant(development of new BM). Categorical and continuous covariates were tested by Fisher exact or Mann-Whitney test, respectively. OS by Kaplan-Meier with groups compared by log-rank. For each model the Harrell Concordance Index(CI) was performed.
4c3880bb027f159e801041b1021e88e8 Result
From 41 eligible patients with BM, 9 were excluded due to sequencing quality. Of the 32 remaining patients, 20 (62%) had their BM treated with WBI (15 WBI alone and 5 TKIàWBI), 12 (38%) with TKI±SRS (9 TKI àSRS; 2 TKI alone and 1 SRS alone). Median age at BM was 59.5 years(y). Most of the cohort were female(81%), non-smoker(78%), non-Asian(62%) and 50% presented as stage III or higher at diagnosis. An EGFR exon 19 mutation was present in 72% of patients, 25% had 2 or more EGFRm, 15% with additional driver mutations and 53% with TP53 co-mutation. At a median follow-up of 1.21-y, no clinical/molecular factors(treatment, age, gender, ethnicity, smoking status, stage at presentation, EFGR exon 19 versus 21, number of EGFRm, additional driver mutations, TP53 co-mutation) correlated with survival. There was a trend for longer survival for patients treated with TKI±SRS(median 3.4y) compared to WBRT±TKI(median 1.4y); p=0.08 and for age at BM ≤59.5y(median 2.5y) compared to >59.5y (median 1.4y); p=0.2. Higher risk of IP was observed in younger patients (age as continuous variable) with HR of 0.94(95%CI 0.88-1.0), p=0.04; favoring older patients and remained significant after accounting for treatment modality on multivariate analysis p=0.03. No additional clinical/molecular factors correlated with IP.
8eea62084ca7e541d918e823422bd82e Conclusion
In our study, younger age at BM treatment was associated with higher IP. We also observed a trend for longer OS for younger patients(≤59.5y) and for patients treated with TKI±SRS. Our data suggest that younger patients with EGFR BM should undergo close intracranial follow up and that future studies to define the benefit of brain-directed multimodality treatment are warranted.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA27 - Novel Drugs and PDX Models (ID 931)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 13:30 - 15:00, Room 206 BD
-
+
MA27.03 - Multi-Omic Characterization of TKI-Treated Drug-Tolerant Cell Population in an EGFR-Mutated NSCLC Primary-Derived Xenograft (ID 13370)
13:40 - 13:45 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Background
Sixty to eighty percent of advanced stage lung adenocarcinoma patients with epidermal growth factor receptor (EGFR) mutated tumors respond to first generation EGFR tyrosine kinase inhibitors (TKIs). However, cure is not yet achievable with any EGFR TKI monotherapy, as patients eventually progress due to acquired resistance. In vitro evidence suggests that minor populations of epigenetically modified drug tolerant cells (DTCs) may be important for tumor cells surviving TKI. We hypothesize that molecularly characterizing DTCs in vivo and comparing them to the untreated tumor in a patient-derived xenograft (PDX) model may delineate mechanisms of tolerance that closely mimic those occurring in patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
DTCs were produced via chronic exposure to erlotinib in a lung adenocarcinoma PDX harbouring an exon 19 deletion. Histological, genomic, transcriptomic (including single-cell RNA-seq), and epigenetic characterizations were performed on DTCs and compared to untreated baseline (BL) tumors.
4c3880bb027f159e801041b1021e88e8 Result
Compared to BL, DTCs exhibit decreased levels of proliferation (Ki67 by immunohistochemistry (IHC) and increased expression of senescence/quiescence (p21) and anti-apoptosis (BCL-XL) immunohistochemistry (IHC) markers, while maintaining EGFR pathway signaling (pEGFR, pAKT, pERK, pS6 IHC). Whole exome-sequencing provides evidence that DTCs likely do not represent mutationally distinct subclones from the bulk tumor. Instead, DTCs exhibit a number of differentially expressed genes compared to BL tumors that are involved in cell cycle arrest, senescence/quiescence, differentiation, vesicles, and inflammation. Genes with epigenetic differences (chromatin openness and/or promoter methylation) are involved in similar cellular processes. A minor (<2%) subpopulation of transcriptomically-defined DTC-like cells in the BL tumors are very similar to the DTCs, supporting the hypothesis that DTCs may exist prior to treatment. A number of transcription regulators are found to have differential gene expression and epigenetic regulation as well as DNA-binding motifs found in regions of chromatin uniquely open in DTCs or baseline tumors. These transcription regulators are involved in cell maintenance, proliferation, and differentiation, and may play key roles in promoting DTC phenotype.
8eea62084ca7e541d918e823422bd82e Conclusion
In this specific EGFR mutant PDX model sensitive to first generation TKIs, DTC-like cells are found in the BL untreated tumors, and its resultant phenotype after exposure to TKI appears to be involved in cell cycle, differentiation, senescence/quiescence, proliferation and maintenance. PDX models may provide insights into therapeutic strategies to target DTCs, and further improve the survival of EGFR-mutated NSCLC patients.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MTE01 - Preclinical Models of Lung Cancer (Ticketed Session) (ID 811)
- Event: WCLC 2018
- Type: Meet the Expert Session
- Track: Biology
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 07:00 - 08:00, Room 206 F
-
+
MTE01.02 - Lung Patient Derived Xenograft and Organoid (ID 11547)
07:30 - 08:00 | Author(s): Natasha B Leighl
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 3
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-42 - Real-World Evaluation of Tolerability in Older Adult Patients (≥75 Years Old) with EGFR-mutated NSCLC (ID 13289)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
NSCLC patients carrying EGFR mutations are diagnosed across a wide age distribution. Although EGFR tyrosine kinase inhibitors (TKIs) are generally well tolerated, there remains a paucity of real-world data on toxicity and health utility scores (HUS) in older patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
A longitudinal observational study evaluated health-related quality of life (HRQoL) using HUS through the EQ-5D questionnaire, and common EGFR-TKI toxicities using PRO-CTCAE in NSCLC outpatients carrying EGFR mutations. Patients were classified into two groups: older (>75 years) and younger (<75 years). Patient characteristics and outcomes were extracted from chart review; patients were classified as having stable or progressive disease according to imaging findings. HUS and PRO-CTCAE results were compared descriptively.
4c3880bb027f159e801041b1021e88e8 Result
Of 240 patients and 774 encounters, 52 patients (22%; comprising 157 encounters) were aged ≥ 75 years. Gender and race were similarly distributed in both age groups: 63% of older patients and 70% of younger (<75 years) were female; 56% of older patients and 53% of younger patients were Asian. Use of gefitinib in older patients was much higher than other drugs: among 147 patients who received gefitinib, 27% (40 patients) were older, compared to 15% (5/33) for osimertinib and 15% (3/20) for erlotinib. Of patients receiving afatinib (n=11) and chemotherapy (n=32), none were ≥ 75 years. The following table describes HUS and PRO-CTCAE results by treatment and age group for stable patients.
Older Adults (≥75 years)
Younger Adults (<75 years)
N
HUS, mean (SD)
PRO-CTCAE*, median [IQR]
N
HUS, mean (SD)
PRO-CTCAE*, median [IQR]
Stable on gefitinib
34
0.83 (0.20)
4.5 [0,16]
77
0.80 (0.15)
4 [0,15]
Stable on osimertinib
5
0.80 (0.23)
13.5 [0,17]
22
0.87 (0.12)
0 [0,13.5]
Stable on erlotinib
3
0.82 (0.08)
0 [0,9]
11
0.80 (0.14)
0 [0,16]
*Higher PRO-CTCAE indicates more severe toxicities/symptoms.
8eea62084ca7e541d918e823422bd82e Conclusion
In a real-world evaluation, patients 75 years or older comprised almost a quarter of all patients with EGFR-mutant advanced NSCLC. Afatinib and chemotherapy were not used at all in this population. Gefitinib was used most commonly, with similar toxicities and health utilities between older and younger patients. Osimertinib and erlotinib were used too infrequently in this study for conclusive age comparisons.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-52 - BR-34- Randomized Trial of Durvalumab & Tremelimumab +/- Platinum Chemotherapy in Patients with Metastatic Squamous or Non-Squamous NSCLC (ID 11791)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Background- Immunotherapy improves survival of patients with non-small cell lung cancer (NSCLC). Current clinical trials are studying various combinations of PD-1/PD-L1 inhibitors and CTLA-4 agents with or without chemotherapy, to enhance treatment efficacy. This trial will determine the effects of adding platinum chemotherapy to combination of check point blockade with durvalumab and tremelimumab in the first line treatment of advanced non-small cell lung cancer.
a9ded1e5ce5d75814730bb4caaf49419 Method
Method- BR-34 is a CCTG led randomized proof-of-concept trial of durvalumab and tremelimumab, with or without platinum-based chemotherapy in patients with metastatic squamous or non-squamous NSCLC. Patients who have histologically confirmed Stage IV NSCLC and wild type EGFR and ALK, PDL-1 unselected, with measurable disease by RECIST 1.1, and available tissue for biomarker testing are eligible and are stratified by stage, histology and smoking status. Primary end point is overall survival. Secondary end points include progression free survival at 1 year, overall response rate, quality of life, cost effectiveness and correlative studies on tissue and blood (including PD-L1, tumour mutation burden and cell-free DNA) with outcomes and response, and PFS by iRECIST (exploratory). In total 300 patients will be recruited from Canada, Australia and Italy. Arm A will receive 4 cycles of fixed doses of tremelimumab (T) 75 mg plus durvalumab (D) 1500 mg every 28 days IV, followed by durvalumab (D) maintenance and Arm B will receive standard platinum-doublet chemotherapy in combination with T+D every 21 days for 4 cycles, followed by maintenance D (with pemetrexed for those with non-squamous histology), until progression.
4c3880bb027f159e801041b1021e88e8 Result
Results- BR-34 was initiated in February 2017 in Canada and total 150 patients have been randomized as of April 2018, including 137 from Canada and 13 from Australia. 28 sites are open to accrual in Canada, 15 in Australia the trial will soon be opened in Italy.
8eea62084ca7e541d918e823422bd82e Conclusion
Conclusion- At the current rate of accrual, the CCTG BR-34 trial should be fully accrued by Q1 2019. This is the first randomized trial of combination checkpoint blockade +/- platinum-doublet chemotherapy in advanced non-small cell lung cancer.
Acknowledgements: BR-34 is an academic, co-operative group trial led by Canadian Cancer Trials Group (CCTG) in collaboration with ALTG and NHMRC Clinical Trials Centre, with support from AstraZeneca and Canadian cancer society. NCT03057106
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-54 - A Phase I/Ib Study of Binimetinib (MEK162), a MEK Inhibitor Plus Carboplatin/Pemetrexed in Non-Squamous NSCLC (ID 14205)
16:45 - 18:00 | Presenting Author(s): Natasha B Leighl
- Abstract
Background
MEK remains an important potential target in RAS/RAF dependent tumours. Binimetinib (MEK16) is a highly selective oral inhibitor of MEK1/2, which has shown activity in melanoma and other cancers. Data from preclinical models suggest greater activity with intermittent versus continuous dosing of MEK inhibitors when added to chemotherapy. This dose escalation study combines intermittent binimetinib with pemetrexed plus carboplatin in chemotherapy naive patients with advanced non-squamous NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
A standard 3+3 dose-escalation design was used with planned dose expansion at RP2D (N=30) in genotypically defined cohorts (KRAS G12C mutant (mt), non-G12C mt and wild type). The primary endpoint was to define the RP2D of binimetinib using an intermittent dosing schedule in combination with standard doses of pemetrexed and carboplatin. Secondary objectives included safety, pharmacokinetics (PK), response and exploring potential biomarkers of response or toxicity. Binimetinib (30-45 mg BID) was commenced on day 1 cycle 1 for 5 days followed by a 48-hour washout period. If well tolerated, pemetrexed/carboplatin (500 mg/m2/AUC 5 mg*min/mL) were added on day 8 cycle 1 and binimetinib resumed from days 8-26 with a 48 hour drug washout before cycle 2. For subsequent cycles, pemetrexed/carboplatin were administered on day 1, binimetinib on days 1-19 every 21 days. Key eligibility criteria included ECOG performance status 0-1, adequate organ function, measureable disease by RECIST and no active CNS metastasis. Patients with EGFR/ALK mt lung cancer were permitted in the dose escalation phase after progression on standard TKI therapy.
4c3880bb027f159e801041b1021e88e8 Result
Between 03/2017 and 04/2018, 11 patients have been enrolled in 2 dose escalation cohorts (4 KRAS mt, 4 EGFR mt including 1 ex20ins). Six patients were treatment-naive, 3 received prior EGFR TKI and 2 prior pembrolizumab. To date, 7 have experienced grade 3 adverse events (AEs) deemed at least possibly related to binimetinib (anemia, neutropenia, abdominal pain, diarrhea, nausea, vomiting, fatigue, transaminitis). No grade 4/5 AEs have been seen. Related AEs seen in >20% of patients include: rash, mucositis, diarrhea, fatigue, transient visual changes, nausea, edema, neutropenia and vomiting. Antitumour activity was seen at each dose level of binimetinib. Final data on toxicity, response, PK and confirmation of RP2D will be presented at the meeting.
8eea62084ca7e541d918e823422bd82e Conclusion
The addition of binimetinib to pemetrexed/carboplatin appears to have manageable toxicity with evidence of activity in non-squamous NSCLC. Dose expansion in genotypically defined cohorts will continue and a cohort combining binimetinib with pembrolizumab is planned.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-11 - PRO-CTCAE Toxicities in Advanced NSCLC Patients with EGFR Mutations: A Real World Assessment (ID 12998)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
The Patient Reported Outcomes of the CTCAE (PRO-CTCAE) tool has not been evaluated in a real-world study of EGFR-mutation positive patients treated with TKIs/chemotherapies. We evaluated its role in capturing clinically-significant toxicities.
a9ded1e5ce5d75814730bb4caaf49419 Method
A longitudinal observational study evaluated common EGFR-TKI toxicities using PRO-CTCAE, measured on a five-point scale (1=no symptoms to 5=very severe symptoms) in outpatients with EGFR-mutated (EGFRm) advanced NSCLC.
4c3880bb027f159e801041b1021e88e8 Result
Toxicity information was collected for 709 follow-up visits (encounters) from 232 patients. Median age was 64 (range:29-96), 161 (69%) were female and 124 (53%) were Asian. 85 (37%) already had brain metastases at first encounter. 485 encounters were observed from patients stable on treatment, and 187 from patients progressing or with documented progression on their current treatment. 24 patients were treated with osimertinib (97 encounters, 97% in second/subsequent-line), 136 with gefitinib (324 encounters, 95% in first line therapy), 42 were receiving other EGFR-TKIs (118 encounters, 53% in second/subsequent-line), and 29 with chemotherapy (73 encounters, 96% second/subsequent-line). The table below summarizes the treatment-related PRO-CTCAE toxicities self-graded as moderate-to-very-severe by EGFRm patients.
Proportion of patients reporting highest grade of toxicity as grade 3-5, by PRO-CTCAE
Gefitinib
Osimertinib
Other EGFR TKI
Chemotherapy
Diarrhea
17%
18%
24%
8%
Constipation
12%
4%
12%
16%
Decreased appetite
10%
7%
14%
26%
Nausea
6%
3%
4%
24%
Vomiting
1%
2%
3%
16%
Fatigue
18%
12%
23%
42%
Numbness and Tingling
6%
7%
10%
16%
Skin Rash
23%
12%
20%
9%
Visual Disorders
(includes dry eye)
4%
0%
3%
4%
Total PRO-CTCAE Score, MEDIAN [IQR]
4 [0,16]
0 [0,15]
6 [0,17]
10 [0,21]
8eea62084ca7e541d918e823422bd82e Conclusion
Osimertinib therapy had the most favorable self-reported toxicity profiles of all the therapies in EGFRm patients, followed by gefitinib. Chemotherapy generated the greatest toxicities. The use of PRO-CTCAE was well-accepted by patients in a clinical setting. This confirms trial data supporting favorable toxicities with osimertinib compared to other therapies for EGFRm NSCLC patients.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.13-15 - Detection of EGFR Mutations in cfDNA and Development of Resistance (ID 12634)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Peripheral blood sampling for T790M in patients (pts) failing initial EGFR-TKIs is now standard practice. The value of longitudinal sampling in pts is unknown.
a9ded1e5ce5d75814730bb4caaf49419 Method
A study of cell free (cf) DNA analysis in pts with EGFR mutated(m) non-small cell lung cancer (NSCLC) is ongoing at the Princess Margaret Cancer Centre. The ThermoFisher OncomineTM lung assay detecting single nucleotide variants and indels to a limit of 0.05-0.1% variant allele frequency (VAF) was used. Patient clinical details and outcomes were collected prospectively.
4c3880bb027f159e801041b1021e88e8 Result
From Oct 2016-Feb 2017, 73 pts with EGFRm NSCLC enrolled and first blood samples were analysed. Most (92%) had mutations in del19 or L858R, including 1 pt with del19/S768I. Uncommon EGFRm were present in 6 (G718X, L861Q, exon20ins). Detectable levels of cfDNA were found in 50 pts (68%). Of 64 pts either starting an EGFR-TKI (n=11,17%), receiving a TKI without progression (PD) (23, 36%) or with PD on a TKI (30, 47%), the presence of the primary EGFRm (n=39, 61%) strongly associated with pre- 1st TKI or PD, p=0.03. Of 53 pts receiving a TKI, the presence of T790M in 31 (58%) associated with PD (p=0.04). Where pts had no radiologic PD evident, the median progression free survival (PFS), taken from blood draw, was 2.1 months (mths) versus 10 mths (HR 2.22, 95% CI: 0.89-5.54 p=0.08) when the primary EGFRm was detected. If T790M was present in cfDNA, the median PFS was 3.0 months versus 9.7 mths, (HR 4.59, 95% CI: 1.43-14.73 p=0.005). In univariable regression analyses the %VAF of the primary EGFRm correlated with PFS (HR 1.15, 95%CI: 1.02-1.29, p=0.02) with a trend in the %VAF of T790M (HR 1.16 95% CI:0.99-1.37, p=0.08). T790M was detected in 3 of 4 pts with T790M -ve tissue, and other co-occurring EGFRm were found in 10 pts including K745R in a pt receiving first-line osimertinib. TP53 (n=10), KRAS (1), PI3KCA(1) and ALK(2)gene mutations were also detected. Interestingly, in 1 pt receiving chemotherapy with T790M+ disease, both the primary EGFRm and T790M were detected in blood at the time of PD.
8eea62084ca7e541d918e823422bd82e Conclusion
In addition to the emergence of resistance mutations, the presence of the primary EGFRm in pts receiving EGFR-TKIs may associate with a shorter PFS and therefore may be useful in longitudinal analyses of cfDNA to direct therapy.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 4
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-03 - Quality of Life Outcomes for FDA-Approved Agents in Advanced Non-Small Cell Lung Cancer (ID 14214)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Symptom control and quality of life (QoL) improvement are among the most important goals of systemic therapy in advanced NSCLC, but they are not required for regulatory approval. We reviewed published QoL data for agents approved for use in advanced NSCLC by the United States Food and Drug Administration (FDA) and explore their potential uses in the era of precision medicine.
a9ded1e5ce5d75814730bb4caaf49419 Method
Through systematic review, we identified 19 agents approved by the FDA for the treatment of advanced NSCLC between 1999 and 2017. Clinical trials associated with regulatory approval of these agents were identified. QoL results were abstracted from study publications, in addition to trial characteristics (phase, sponsor).
4c3880bb027f159e801041b1021e88e8 Result
46 trials were identified supporting the FDA approval of the 19 agents. Of these, 33 (72%) were phase 3 randomized trials (including 3 cooperative group studies) and 13 (28%) were early phase (I/II) trials. QoL data were reported for 27 trials (59%), including all cooperative group trials. Another 5 (11%) measured QoL but results were not published. The remaining 13 (24%) trials did not measure QoL, 8/13 are phase III, 3/13 phase II and 2 phase I. In trials that reported QoL outcomes, better global QoL and cancer-related symptoms were identified in 17/33 (51%) and 18/33 (55%), respectively. Time to symptom deterioration was reported in 18 (55%) studies, with all but one showing benefit for the approved agent. The remaining studies reported no significant difference in QoL between study treatment arms. All studies reporting improved QoL parameters also reported improved response rates. In trials demonstrating OS and PFS improvement, 3/14 and 10/21 reported better QoL outcomes, respectively. ORR was higher in 26 trials, and it was associated with improvement in QoL in 12/26. Of 12 early phase trials leading to drug approval based on response rate and duration, 6 reported improved QoL outcomes. Three have not yet reported QoL data and 3 did not measure QoL.
8eea62084ca7e541d918e823422bd82e Conclusion
QoL is clearly associated with response rate but improved QoL or symptom control was only demonstrated in17/33 (51%) and 18/33 (55%) of studies supporting regulatory approval. QoL may be a clinically relevant endpoint for drug approval based on early phase trials of agents with high response rates and should be given further consideration by pharmaceutical sponsors and regulators in this era of precision medicine.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.01-29 - <sub>Economic Analysis of Osimertinib in Previously Untreated EGFR-Positive Advanced Non-Small Cell Lung Cancer</sub> (ID 13592)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Osimertinib doubles progression-free survival (PFS) in previously untreated EGFR-positive advanced non-small cell lung cancer (NSCLC) patients, with remarkable intracranial response rates. However, its cost-effectiveness has not been established. We assessed the cost-effectiveness of first-line osimertinib from the perspective of the Canadian public health care payer.
a9ded1e5ce5d75814730bb4caaf49419 Method
A remaining lifetime Markov model was used to project the outcomes and costs of 2 treatment pathways, osimertinib or current standard-of-care (SoC) first-line EGFR TKI gefinitib or afatinib, in previously untreated EGFR-mutant advanced NSCLC patients from the health care system perspective. Clinical, health preference and cost input estimates were informed from the available literature, including second-line osimertinib after SoC failure in those with EGFR T790M mutant cancer. Model outcomes included costs (in 2017 Canadian dollars), quality-adjusted life-years (QALYs), and the incremental cost per QALY gained. The model was fully probabilistic to assess parameter uncertainty.
4c3880bb027f159e801041b1021e88e8 Result
Osimertinib was associated with a gain of 0.70 quality-adjusted life-years (QALYs) at an incremental cost of $58,619 vs SoC (incremental cost-effectiveness ratio [ICER]: $83,164/QALY gained). Unadjusted LY gain was 0.95. Osimertinib had a 7% probability of being cost-effective at a willingness-to-pay threshold of $50,000/QALY, and a 77% probability at a threshold of $100,000/QALY. Deterministic sensitivity analysis showed that health utilities and cost of osimertinib had the largest impact on ICER results.
8eea62084ca7e541d918e823422bd82e Conclusion
First-line osimertinib use in patients with advanced EGFR mutant lung cancer was found to involve a trade-off between improved PFS, QALYs and LYs versus increased cost.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.01-46 - Investigating the Effects of Prior Malignancy on NSCLC Trial Eligibility (ID 12617)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Up to 18% of patients with non-small cell lung cancer (NSCLC) who are candidates for participation in a clinical trial are excluded due to a prior malignancy. Common reasons include the need for another anti-cancer therapy and potential impact on clinical trial endpoints. We evaluated the proportion of trials that exclude patients with a prior history of malignancy, and estimate the potential impact of the prior malignancy from a cohort of NSCLC patients treated with first line systemic therapy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Clinical trials of palliative systemic therapy for NSCLC registered on or after January 1st 2014 from clinical trials.gov were reviewed to determine the proportion of trials that included a prior malignancy as an exclusion criterion. Patients with NSCLC and a prior malignancy, treated with first line palliative systemic treatment between January 1st 2010 and January 1st 2016 at the Princess Margaret Cancer Centre (PMCC), Toronto, Canada were identified from a cancer registry. A list of prespecified criteria of ways in which a prior malignancy may impact safety or a clinical trial endpoint was defined. These included: requirement for non-NSCLC anticancer therapies, other cancer detected on imaging, biopsy or tumour markers, disease progression or death from other malignancy, clinician uncertainty as to presence of synchronous other cancers. The proportions of patients with one or more of these criteria were assessed.
4c3880bb027f159e801041b1021e88e8 Result
66% of 359 clinical trial protocols contained an exclusion criterion for prior malignancy. 112 patients with advanced NSCLC and a prior malignancy were identified at PMCC, the median age was 71, 49% were male, the median time between prior cancer and NSCLC diagnosis was 74 months. 21% of patients were enrolled on a clinical trial. 52(59%) of the 88 patients not enrolled on a clinical trial did not meet any of our predetermined criteria, which may impact safety or a clinical trial endpoint due to other malignancy.
8eea62084ca7e541d918e823422bd82e Conclusion
A prior history of a malignancy is a common exclusion in NSCLC clinical trials. Preliminary results suggest the majority of patients in our study did not appear to have a complication that would have impacted safety or clinical trial endpoints. Further analyses to explore appropriate exclusion criteria for prior malignancies, which may minimize need to exclude patients from clinical trials, are underway.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.01-94 - Diagnostic Patterns of Non-Small Cell Lung Cancer at Princess Margaret Cancer Centre (ID 14178)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Accurate classification of lung cancer subtypes has become critical in tailoring lung cancer treatment. Our study aimed to evaluate changes in diagnostic testing and pathologic subtyping of advanced non-small cell lung cancer (NSCLC) over time at a major cancer centre.
a9ded1e5ce5d75814730bb4caaf49419 Method
A review of patients diagnosed with advanced NSCLC at the Princess Margaret Cancer Centre between 2007-2009 and 2013-2015 was performed. Diagnostic method, sample type and site, pathologic subtype, and use of immunohistochemical (IHC) staining and molecular testing were abstracted.
4c3880bb027f159e801041b1021e88e8 Result
A total of 238 patients were reviewed in 2007-2009 and 283 patients in 2013-2015 (Table 1). Over time, the proportion of patients diagnosed with adenocarcinoma increased from 60.9% to 73.1% while NSCLC-not otherwise specified (NOS) diagnosis decreased from 18.9% to 6.4%, p<0.0001. There was a decrease in use of diagnostic bronchoscopy (26.9% vs 18.4%) and an increase with mediastinal sampling procedures including endobronchial ultrasound (9.2% vs 20.5%), p=0.0001. A substantial reduction in cases reported as NSCLC-NOS was observed among bronchoscopy, image-guided, and mediastinal sampling procedures. The reduction in NSCLC-NOS was also predominantly seen in cytology samples, from 22.0% to 4.0% (p<0.0001).
IHC use increased over time from 41.6% to 76.3% (p<0.0001). Patients with larger samples and IHC analysis were more likely to have biomarker testing performed (both p<0.01). Within the group diagnosed with NSCLC-NOS, the use of IHC increased non-significantly from 64% (29/45) to 94% (16/18). With the exception of bronchoscopy samples, use of IHC increased significantly with each method of diagnosis and sample type.
8eea62084ca7e541d918e823422bd82e Conclusion
Customizing treatment based on pathologic subtype and molecular genotype has become key in treating advanced lung cancer patients. Greater accuracy of pathologic diagnosis is being achieved including through use of routine IHC.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.03 - Biology (Not CME Accredited Session) (ID 952)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.03-03 - Upfront Next Generation Sequencing in NSCLC: A Publicly Funded Perspective (ID 11826)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
A growing number of targeted drug treatments in non-small cell lung cancer (NSCLC) have led to the need for molecular profiling beyond the standard of care (SOC) EGFR/ALK. Here we present actionable targets, impact on patient treatment, clinical trial opportunities and costs using the Illumina TruSight Tumor 15 panel (TST15) for NSCLC samples.
a9ded1e5ce5d75814730bb4caaf49419 Method
Tissue-based next generation sequencing using the TST15 was reflexively performed on all newly diagnosed cases of non-squamous NSCLC at the University Health Network (Toronto, Canada) from February 2017-February 2018. The panel identifies hot spot mutations in KRAS, EGFR, TP53, PIK3CA, BRAF, ERBB2, FOXL2, GNA11, GNAQ, KIT, NRAS, PDGFRA, RET, AKT1 and MET, but not fusions, copy number variations (CNV) nor MET exon 14 skipping mutations. Patient age, stage, pathologic subtype, and genotyping results were collected prospectively. Treatment changes as a result of TST15 and clinical trial opportunities (clinicaltrials.gov) were identified. Incremental testing costs were based on direct laboratory costs, but not personnel and administration costs.
4c3880bb027f159e801041b1021e88e8 Result
Testing included 342 samples from 336 patients. The TST15 panel identified 409 mutations from 342 samples. Sample demographics include: male: 53, and stage 1/2/3/4: 34/8/15/43%. Incremental actionable targets beyond EGFR and ALK were identified in 3.5% of patients (ERBB2 2.3%, BRAF V600E 1.2%). Most mutations occurred in TP53 (43%), EGFR (24%) and KRAS (26%). Co-mutations occurred in 32% (TP53, KRAS, EGFR) of samples. To date, one patient has had a treatment change as a result of TST15 beyond targeting EGFR. Above SOC clinical trial options were identified for 88% of stage IV and 26% of stage III patients. 3.6 samples were needed to identify one actionable mutation, predominantly in EGFR, at an estimated cost of $1919 CAD per target.
8eea62084ca7e541d918e823422bd82e Conclusion
Extended genotyping with TST15 in NSCLC identifies an additional 3.5% of patients with actionable mutations above SOC and improves clinical trial options for patients. Despite this, impact on patient treatment beyond targeting EGFR is minimal. To enhance the number of targets and minimize costs, affordable population-based comprehensive testing with a panel that includes fusions/CNV is needed.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.03-04 - Next Generation Sequencing in Lung Cancer Using the Oncomine Comprehensive Assay: The Canadian Publicly Funded Experience (ID 12181)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Standard of care (SOC) diagnostics for patients with stage IV non-small cell lung cancer (NSCLC) in Canada includes EGFR and ALK testing. Other genomic alterations are not tested routinely; however, access to enhanced molecular testing may broaden treatment options, clinical trial access, and improve outcomes for patients. This study uses the Oncomine Comprehensive Assay (OCA) v3, a next generation sequencing (NGS) panel in NSCLC to evaluate actionable targets, clinical trial eligibility, treatment impact, costs, turnaround time, and patient preference.
a9ded1e5ce5d75814730bb4caaf49419 Method
Consecutive consenting stage IV NSCLC outpatients at the Princess Margaret Cancer Centre without EGFR/ALK/KRAS/BRAF derangement diagnosed at the University Health Network (UHN) in Toronto, Canada will be enrolled to undergo OCA testing on diagnostic samples. The selected platform (OCA v3, ThermoFisher) includes 161 genes including hotspots, fusions, and copy number variations. Patient age, pathologic subtype, genotyping results and treatment history will be collected. Primary endpoints include incremental actionable targets identified and clinical trial opportunities (clinicaltrials.gov) added through incremental testing beyond SOC. Secondary endpoints include treatment changes as a result of OCA testing, costs from the perspective of the Canadian public healthcare system, patient willingness-to pay, and test turnaround time.
4c3880bb027f159e801041b1021e88e8 Result
The study activated in February 2018 with 7 patients enrolled as of April 2018. Results of the value of incremental OCA testing beyond standard of care in the Canadian public healthcare system will be presented at the meeting.
8eea62084ca7e541d918e823422bd82e Conclusion
While OCA testing in patients with advanced NSCLC may identify more actionable targets than selected genotyping, its cost effectiveness in the Canadian healthcare system is unknown and will be determined through this study.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.06 - Mesothelioma (Not CME Accredited Session) (ID 955)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.06-23 - Association of Two BRM Promoter Polymorphisms and Tobacco Exposure with Malignant Pleural Mesothelioma (MPM) Risk and Survival (ID 12738)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Brahma (BRM) is a critical ATPase subunit of the SWI/SNF chromatin remodeling complex. Homozygous 6-7bp insertion variants at two polymorphic promotor sites (BRM-741/BRM-1321) cause epigenetic suppression of BRM expression and they have been reported as susceptibility and/or prognostic markers in many malignancies, including non-small cell lung cancer. As epigenetic silencing of BRM can be reversed pharmacologically, targeting BRM polymorphisms has significant therapeutic potential. We evaluated BRM-741/BRM-1321 polymorphisms’ association with risk and prognosis of malignant pleural mesothelioma (MPM). While the effect of tobacco in MPM remains controversial, its interaction with genetic polymorphisms in MPM is largely unknown. We evaluated associations between BRM polymorphisms-tobacco exposure and MPM.
a9ded1e5ce5d75814730bb4caaf49419 Method
MPM and age-distribution matched asbestos-exposed controls were recruited as part of an asbestos-exposure surveillance program. Participants were genotyped for BRM polymorphisms. Multivariable logistic regression assessed risk of MPM in case-control analyses. Association of BRM variants with overall survival (OS) was assessed by multivariable Cox regression. Secondary subset analyses were performed, stratified by smoking status.
4c3880bb027f159e801041b1021e88e8 Result
Of 265 MPM cases, 146 (55%) were ever-smokers; 51 (19%) were female; median age was 66 (range:21-84) years. Median OS was 18 months; median follow-up time was 15 months. Compared to 795 controls, there were no significant associations of BRM with risk (P>0.10, all comparisons). In contrast, compared to the wild-type genotype, the homozygous variant of BRM-741 and BRM-1321 were associated with lower OS, with adjusted hazard ratio (aHR)=2.56 [95%CI:1.7-3.8] and 2.07 [95%CI:1.4-3.1], respectively. Compared to patients carrying the double wild-type, patients homozygous at both loci had lower OS (aHR=2.70 [95%CI:1.7-4.4]).
There was a significant differential effect of BRM polymorphisms on risk of MPM, by smoking status (interaction P<0.001): among ever-smokers, BRM homozygous variants in BRM-741, BRM-1321, or both conferred lower risks (adjusted odds ratios, aORs were between 0.18-0.28; each P<0.001), while for never-smokers, there was significantly greater risk conferred by carrying the homozygous variants (aORs between 2.7-4.4). Likewise, a similar differential effect by smoking status was seen in prognosis (interaction P<0.001): there was no association between BRM polymorphisms and OS in ever smokers (P>0.1, all comparisons), but in never-smokers, the aHR of carrying homozygous variants of BRM-741, BRM-1321 or both were 7.7 [95%CI:3.8-16], 4.0 [95%CI:2.1-7.7], and 8.6 [95%CI:3.7-20], respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
Never-smokers who develop malignant pleural mesothelioma have an increased chance of carrying BRM homozygous variants in their germline DNA, which results in substantially worse prognosis. In contrast, in smokers, there may be a protective effect, with no difference in overall survival.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 964)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.15-10 - Determinants of Health Utility Scores (HUS) in Patients with ALK Rearranged Non-Small Cell Lung Cancer. (ID 14025)
16:45 - 18:00 | Author(s): Natasha B Leighl
- Abstract
Background
Despite an increasing number of available treatment options for patients with advanced ALK-rearranged (ALKr) NSCLC, the goals of care remain to improve survival whilst maintaining quality of life. With rapidly changing management and prognosis, understanding the impact of treatments on quality of life becomes important. We evaluated factors affecting health utility scores (HUS) in this generally younger cohort of patients with NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
An ongoing prospective observational/real-world cohort study at the Princess Margaret Cancer Centre is enrolling patients with advanced NSCLC. Patients are asked to complete the EQ5D questionnaire at each outpatient visit. During the period from November, 2014 to March, 2018, patients with ALKr who participated were analyzed. Baseline clinical characteristics, oncological treatment, and outcomes were extracted from chart review. Factors associated with HUS were identified using ANOVA.
4c3880bb027f159e801041b1021e88e8 Result
272 encounters (follow-up visits) in 43 ALK+ patients were analysed. The median age at diagnosis was 56 years (range: 31-79 years). Half the patients in our sample were female (49%), and the majority were never-smokers (84%); 44% of the cohort were Asian. During the study period, 24 patients (56%) presented with or developed brain metastases. The median number of HUS collected per person was 4 (range: 1-20). Among patients who were stable on treatment, there were no statistically significant differences in mean HUS (mHUS) between different ALK inhibitors: mHUS [SD, standard deviation] were: mHUS of crizotinib=0.82 [0.08] (n=25, 92 encounters); mHUS of ceritinib=0.79 [0.14] (n=17, 102 encounters); and mHUS of other ALK inhibitors=0.80 [0.17] (n=12, 25 encounters), which included mHUS of alectinib=0.79 [0.20] (n=8, 10 encounters). The number of previous lines of treatment did not impact HUS (p=0.85). Patients stable on treatment who received prior whole brain radiation (n=14) had lower mHUS compared to those who never had WBRT (mHUS=0.78 [0.14] vs. 0.84 [0.10]; p=0.05), which may partly explain differences in mHUS by sex (64% of patients who had received WBRT were female vs 41% who did not receive WBRT): males=0.84 [0.10] vs females=0.78 [0.14], p=0.04. In 11 stable patients who had received radiation to bone metastases mHUS was 0.75 [0.16] vs. 0.84 [0.09] in those who did not (p=0.006).
8eea62084ca7e541d918e823422bd82e Conclusion
In this study, mean HUS were similar across different ALK inhibitors. Female patients and those receiving whole brain radiation or radiation to bone had lower HUS. This highlights specific factors that influence health-related quality of life in this subset of patients with advanced NSCLC
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.03 - Biology (Not CME Accredited Session) (ID 969)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.03-29 - The Prognostic Effect of Tumor Mutation Burden and Smoking History in Resected EGFR Mutant Non-Small Cell Lung Cancer (ID 13199)
12:00 - 13:30 | Author(s): Natasha B Leighl
- Abstract
Background
Although EGFRm NSCLC occurs mainly in non-smoking patients, most series report 20%-35% of cases in current or previous smokers. Broad molecular profiling of EGFRm NSCLC in smokers has not been reported.
a9ded1e5ce5d75814730bb4caaf49419 Method
Surgically resected primary EGFR exon 19, 20 and 21 mutated NSCLC tumors from 106 patients were molecularly profiled by whole exome sequencing using the Illumina HiSeq2000 platform. Alignment and variant discovery analysis was performed according to GATK best practices workflow; 74 sequenced to a mean coverage of 65.1x. Demographics and outcomes were compared for smokers and non-smokers (non-S), and by mutation profile.
4c3880bb027f159e801041b1021e88e8 Result
Among 53 non-smokers and 21 smokers (5 current/recent within 10 years), 70% were female, 51% non-Asian, 40.5% >65 years of age and 58.1% had EGFR exon 19. Of the 74 patients, 51% were stage I, 19% stage II and 30% stage III+. Smoking was associated with male sex (p= 0.011) and non-Asian ethnicity (p=0.00002) but not age, stage or EGFR exon 19/20/21 subtype. Multiple “driver” mutations occurred in tumors of 23.8% smokers and 26.4% non-S. TP53/EGFR co-mutation occurred in 52.4% smokers and 47.2% non-S. There was no significant difference seen for TMB in smokers: median TMB in smokers 3(1.4-7.46) versus 2.7(0.96-3.95) in non-S (p=0.11). The strongest prognostic factor for OS and DFS was stage (I, II, III+) (p<0.0001 for each compared to stage I). Smoking history did not have a significant effect on survival: HR 1.61 (CI 0.78-3.32, p=0.2) or probability of relapse: HR 0.9 (CI 0.46-1.77, p=0.77). Smoking within 10 years of NSCLC diagnosis was not associated with shorter OS/DFS. Neither EGFRm subtype nor TP53/EGFR co-mutation was associated with probability of relapse or OS. High TMB was significantly associated with shorter survival: HR above vs below the median 2.37 (CI 1.12-5.01, p=0.024), but not probability of relapse: HR 1.67 (CI 0.88-3.19, p=0.12). A subset analysis found the effect of TMB on survival was significant in patients >65 years (p=0.004), but not in patients <65 years (p=0.95).
8eea62084ca7e541d918e823422bd82e Conclusion
Stage remains the strongest prognostic factor for survival and probability of relapse in completely resected EGFRm NSCLC. TMB appears to have an effect on survival outcomes, unlike smoking status, and this effect may be greater in patients older than 65 years.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.04 - Immunooncology (Not CME Accredited Session) (ID 970)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.04-20 - Correlation of Immune-Related Adverse Events and Response from Immune Checkpoint Inhibitors in Patients with Advanced NSCLC (ID 14138)
12:00 - 13:30 | Author(s): Natasha B Leighl
- Abstract
Background
Immune checkpoint inhibitors (ICIs) are associated with a unique set of toxicities termed immune-related adverse events (irAEs). The association between response to ICI therapy and development of irAEs has been documented in various cancer types.
a9ded1e5ce5d75814730bb4caaf49419 Method
Stage IV non-small cell lung cancer (NSCLC) patients treated with ICIs at the Princess Margaret Cancer Centre between 2013 and 2016 were followed for treatment response, treatment duration, survival, and toxicity. The relationship between treatment outcomes and occurrence of irAEs was examined.
4c3880bb027f159e801041b1021e88e8 Result
A total of 97 advanced NSCLC patients were followed. Most patients (81%) received anti-PD-1 agents, 17% received anti-PD-L1 agents, and 2% received combination anti-PD-L1 plus anti-CTLA-4 therapy. Median follow up for the cohort was 5.1 months (0.3-38.1 months) from treatment start. Demographic and tumour characteristics were balanced between the groups. IrAEs occurred in half of patients (51%) on ICIs and grade≥3 irAEs in 7%. The most commonly observed irAEs were arthralgia (13%), diarrhea/colitis (12%), and skin rash (11%). Discontinuation of treatment due to irAEs occurred in 10% of patients, half of whom experienced grade≥3 irAEs.
The overall response rate to ICIs was 23%, with the majority occurring by week 8 of treatment (16/22). Response was non-evaluable in one patient and this was excluded from response analysis. Patients with grade≥3 irAEs were more likely to have response to treatment compared to those with grade I/II irAEs and no irAEs (67% vs 17% vs 23%, p=0.035), Table 1. Smoking status was not associated with response rate or frequency of irAEs. Median survival was not reached in those with grade≥3 irAEs, 15.7 months in those with grade I/II irAEs, and 7.4 months in those with no irAE (p=0.16). Duration of treatment did not differ significantly among the groups.
8eea62084ca7e541d918e823422bd82e Conclusion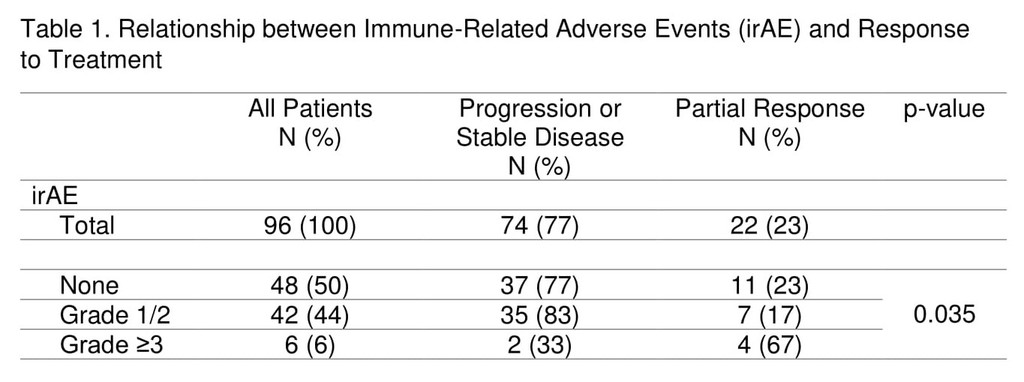
The occurrence of grade≥3 irAEs may be associated with treatment response in advanced NSCLC patients undergoing ICI therapy.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 982)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.16-07 - The Impact of Clinical and Molecular Profile of Resected EGFR-Mutant Non-Small Cell Lung Cancer on the Risk of Developing Brain Metastases (ID 13339)
12:00 - 13:30 | Author(s): Natasha B Leighl
- Abstract
Background
Brain metastases are common in non-small cell lung cancers (NSCLC) with activating EGFR mutations (EGFRm), occurring in 44%-63% of patients. To date, there are no known clinical or molecular factors to predict the risk of brain metastases in these patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
In this retrospective single-institution study, we identified 106 patients with EGFRm NSCLC who underwent surgery for primary lung tumor. Clinical and demographic data was collected from electronic records. Whole Exome Sequencing (WES) of the primary tumor was performed utilizing the Agilent SureSelect Exome v6+COSMIC baits followed by sequencing on the Illumina HiSeq2500 platform. Development of brain metastases was correlated with clinical/pathologic features, EGFR mutation type, co-mutation of EGFR and other frequently mutated genes; and non-synonymous tumor mutation burden (TMB). Statistical analysis used Fisher exact test for categorical variables, Mann-Whitney test for continuous variables of association with the risk of developing brain metastases, and Gray’s test for the probability of brain metastases over time.
4c3880bb027f159e801041b1021e88e8 Result
Of 106 patients who underwent surgical resection of primary EGFRm NSCLC, WES was successful for 73: 51 (70%) females, 52 (71%) never smokers, 38 (52%) stage I, 14 (19%) stage II and 21 (28%) stage III; 42 (57%) EGFR exon 19 mutation, 30 (41%) exon 21, 1(1%) Exon 20 insertion mutation.
Twenty-five patients (34%) developed brain metastases. Patients with brain metastases were younger (median age 61 vs. 65 years, p=0.021), had more advanced stages (p=0.012), with a trend towards higher rates in females (p=0.066). One patient with brain metastases had de-novo EGFR T790M mutation in the primary tumor. No difference was seen regarding smoking history, EGFR mutation type, TP53 co-mutation, and median TMB. The 5-year probability of brain metastases increased with increasing stage (14% stage I; 43% stage II, [HR=3.00], 44% stage III, [HR=3.13], p=0.03), and a trend towards higher probability among females (33% vs. 19%; HR=0.39 for males, p=0.074), and younger patients (37% <65 years vs. 15% >65, HR=0.37 in older patients, p=0.042). There was no difference in probability of brain metastases based on smoking history, ethnicity, EGFR type (33% exon 19 vs. 22% exon 21, p=0.28), TP53 co-mutation (31% vs. 27% without TP53, p=0.59), or TMB (24% TMB≤2.87 vs. 32% TMB>2.87non-synonymous mutations/Mb, p>0.99).
8eea62084ca7e541d918e823422bd82e Conclusion
While our findings suggest that younger age, advanced stage, and female sex may be associated with the development of BM in EGFRm NSCLC, we could identify no molecular predictor of BM based on EGFR subtype, TP53 co-mutation or TMB.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
PL02 - Presidential Symposium - Top 5 Abstracts (ID 850)
- Event: WCLC 2018
- Type: Plenary Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 08:15 - 09:45, Plenary Hall
-
+
PL02.08 - Discussant - PL02.07 (ID 14739)
09:10 - 09:15 | Presenting Author(s): Natasha B Leighl
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
PR01 - Press Conference (ID 872)
- Event: WCLC 2018
- Type: Press Conference
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/23/2018, 16:00 - 17:30, Room 202 BD
-
+
Preview of the WCLC/Key Themes/Practice-Changing Abstracts (ID 14871)
16:05 - 16:13 | Presenting Author(s): Natasha B Leighl
- Abstract
Abstract not provided
-
+
Welcome (ID 13396)
16:00 - 16:05 | Presenting Author(s): Natasha B Leighl
- Abstract
Abstract not provided


