Virtual Library
Start Your Search
Nir Peled
Author of
-
+
MA04 - Novel Approaches with IO (ID 900)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Immunooncology
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 13:30 - 15:00, Room 107
-
+
MA04.03 - Immunotherapy for Non-Small Cell Lung Cancers (NSCLC) with Oncogenic Driver Mutations: New Results from the Global IMMUNOTARGET Registry (ID 13187)
13:40 - 13:45 | Author(s): Nir Peled
- Abstract
- Presentation
Background
Prospective data on immunotherapy for NSCLC with oncogenic driver mutations are limited. We recently reported first results from the global IMMUNOTARGET registry (Mazières, ASCO 2018). Here, we present new data for PD-L1 and mutation subgroups.
a9ded1e5ce5d75814730bb4caaf49419 Method
In 2017, we started an international retrospective registry study ("IMMUNOTARGET") for patients with advanced NSCLC, known driver mutations (KRAS, EGFR, ALK, ROS1, BRAF, HER2, MET and RET) and PD-L1 immune checkpoint inhibitor therapy. The registry is approved by University of Toulouse and Swissethics, and funded by University of Toulouse and Cantonal Hospital of Lucerne. Anonymized real-world data submitted to the coordinating center include: patient and tumor characteristics, mutation test methods and results, systemic therapy lines, immune related adverse events, best response by RECIST, survival, and tumor PD-L1 expression (optional). Statistical calculations including best response, median PFS and OS are done at University of Toulouse.
4c3880bb027f159e801041b1021e88e8 Result
In April 2018, the registry included 551 pts from Europe, USA, Israel and Australia. Patients were 50% male/female, 28% current smokers, median age 60 years (range 28-83), 85% had PS0/1. Most (73%) tumors were stage IV at diagnosis, almost all (96%) were adenocarcinomas. Molecular classification by dominant driver mutation: KRAS=271 (49%), EGFR=125 (23%), BRAF=43 (8%), MET=36 (7%), HER2=29 (5%), ALK=23 (4%), RET=16 (3%), ROS1=7 (1%), 1 (0.2%) not classified (ALK+RET+MET). Most pts received nivolumab (466) or pembrolizumab (48) and were treated with immunotherapy in second or third line (67%). The median number of cycles was 5 (range 1-68). Fifty (11%) pts had grade 3-5 toxicity. Median OS from start of immunotherapy was 13.3 months, median PFS was 2.8 months. Best response was PR/CR in: KRAS=26%, BRAF=24%, ROS1=17%, MET=16%, EGFR=12%, HER2=7%, RET=6%, ALK=0%. Percentage of PD-L1 positive cells was available for 177 pts: 0%=71 (40%), 1-49%=46 (26%), 50-100%=60 (34%). Median % of positive cells was highest for ROS1 (90%), BRAF (50%), MET (30%) and RET (26%) mutant tumors. PD-L1 positivity was predictive for improved PFS in KRAS and EGFR mutant tumors. PD-L1 status was known in 18 tumors with ALK, ROS1 or RET rearrangements: 5 had 0%, 4 had 1-49% and 9 had 50%-100%. No tumor remissions were observed in this subgroup. The registry remains open, updated results will be presented at the conference.
8eea62084ca7e541d918e823422bd82e Conclusion
Although response rates were lower than in KRAS mutant NSCLC, individual tumors with other driver mutations responded to immunotherapy. PD-L1 expression may not accurately predict clinical benefit from immunotherapy in some molecular subgroups, better markers are needed.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA06 - PDL1, TMB and DNA Repair (ID 903)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Biology
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 13:30 - 15:00, Room 206 AC
-
+
MA06.05 - The Micro-Environmental Cross Talk Between Mast Cells and Lung Cancer Cells Through Cell-to-Cell Contact (ID 11140)
14:00 - 14:05 | Author(s): Nir Peled
- Abstract
- Presentation
Background
Mast cells (MCs) are key effectors in allergic reactions, but are also involved in tissue remodeling, wound healing and protection against pathogens. MCs infiltrate tumors and their number within the tumor microenvironment in certain cancer types, such as lung cancer, have been correlated with poor prognosis. The nature of crosstalk between lung cancer and MCs remain poorly resolved. In this study, we investigated the activation patterns within the MCs following cell-to-cell contact with lung cancer cells showing CD73 involvement and implying metabolic changes.
a9ded1e5ce5d75814730bb4caaf49419 Method
Human MCs (HMC-1 and LAD-2) were exposed to Human lung cancer cells (H1299), derived membranes to recapitulate cell contact mediated activation. Lysates of MCs were tested for protein expression and posttranslational modifications (i.e. phosphorylation) by targeted western blotting. We unraveled the intracellular signaling molecules that are necessary for this signaling pathway by a pharmacological approach using several inhibitors. Each condition was repeated at least twice.
4c3880bb027f159e801041b1021e88e8 Result
H1299 membrane exposure activated the ERK 1/2 MAP kinases in HMC-1 and in LAD-2 cells. AKT signaling was also activated in LAD-2 cells as a result of this contact. CD73 dephosphorylates AMP to adenosine within the MCs. Interestingly enough, this ERK 1/2 activation was inhibited by CD73 inhibitor and A3 receptor antagonists in HMC-1 cells. ERK 1/2 activation was inhibited by A3 receptor antagonists and PI3K in LAD-2 cells. Furthermore, we discovered that protein kinase C (PKC) inhibitor augments the activation of ERK 1/2 in LAD-2 cells. In contrast, PKC inhibitor inhibits the activation of ERK 1/2 in HMC-1 cells. In addition, we discovered that the AKT activation was inhibited by A3 receptor and PI3K inhibitors but not by CD 73 inhibitors.
8eea62084ca7e541d918e823422bd82e Conclusion
Our results suggest that H1299 membranes activate ERK 1/2 in HMC-1 cells by a mechanism that involves autocrine formation of adenosine and is mediated by CD 73 and A3 receptor. In addition, we discovered that there is an important difference between the ERK 1/2 MAP kinase signal transduction in HMC-1 and LAD-2 cells, PKC is an inhibitor of the H1299 activation of ERK 1/2 in LAD-2 cells. In contrast, the H1299 membrane activation of ERK 1/2 kinase in HMC-1 cells is mediated by PKC. Furthermore, we can conclude that H1299 membranes activate AKT in an A3 receptor dependent mechanism that is mediated by PI3K.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA25 - Oligometastasis: Defining, Treating, and Evaluating (ID 929)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Oligometastatic NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 13:30 - 15:00, Room 203 BD
-
+
MA25.02 - Searching for a Definition of Synchronous Oligometastatic (sOMD)-NSCLC: A Consensus from Thoracic Oncology Experts (ID 13452)
13:40 - 13:45 | Author(s): Nir Peled
- Abstract
- Presentation
Background
Recent prospective single centre studies reported improved outcomes in patients with sOMD-NSCLC who were treated with radical intent. Since then sOMD has been perceived as a separate disease entity. However, a clear definition of sOMD-NSCLC is lacking. We aimed to develop a definition and diagnostic criteria of sOMD-NSCLC following a consensus process.
a9ded1e5ce5d75814730bb4caaf49419 Method
A European multidisciplinary consensus group was established with representatives from different scientific societies. Consensus questions were extracted from a survey, case series and a systematic review. The questions were discussed, and the statement formulated during a consensus meeting in Dublin (23.01.18).
4c3880bb027f159e801041b1021e88e8 Result
Summary of consensus statement
Defining sOMD-NSCLC
Definition of sOMD is relevant for patients in whom a radical treatment is technically feasible with acceptable toxicity, taking into account all sites, that may modify the course of the disease leading to a long-term disease control.
All sites must be technically and safely treatable.
The maximum number of metastases/organs meeting the criteria involved will depend on the possibility of offering a treatment strategy with radical intent, taking into account local control and toxicity. Based on the systematic review, a maximum of 5 metastases and 3 organs is proposed.
Diffuse serosal metastases and bone marrow involvement are excluded.
Mediastinal lymph node (MLN) involvement should be considered as locoregional disease in the definition of sOMD-NSCLC.
MLN involvement is of importance in determining if a radical local treatment of the primary tumour may be applied and the MLN will not be counted as a metastatic site.
Staging of sOMD-NSCLC
PET-CT and brain imaging are considered mandatory.
In case of a solitary liver metastasis a dedicated MRI of the liver and for a solitary pleural metastasis, thoracoscopy and biopsies of distant ipsilateral pleural sites are advised.
Staging of the mediastinum requires a minimum of a FDG-PET scan, with pathological confirmation preferred if this influences the treatment strategy.
Pathological proof is required unless the MDT decides that the risk outweighs the benefit. Pathology proof is advised for single metastatic location and if it may change the therapeutic strategy, confirmation of the MLN involvement is recommended.
8eea62084ca7e541d918e823422bd82e Conclusion
A multidisciplinary consensus statement on the definition and staging of sOMD-NSCLC was formulated taking into account results of a European survey, a systematic review and case discussion. This statement might be helpful to standardise inclusion criteria in future clinical trials. However, the definition of sOMD may change over time when more prospective data will become available.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.04 - Immunooncology (Not CME Accredited Session) (ID 936)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.04-17 - Tumour Burden as a Predictive Tool of Response to Immune Checkpoint Inhibitors (ICI) in Patients with Metastatic Non-Small-Cell Lung Cancer (ID 12467)
16:45 - 18:00 | Author(s): Nir Peled
- Abstract
Background
ICI are a novel class of agents that have revolutionized treatment for patients with metastatic non-small-cell lung cancer (NSCLC). Still, most patients do not benefit from PD-1 axis inhibitors, emphasizing the need for additional markers beyond PDL-1 expression for better selection of patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
This retrospective, single centre study included all consecutive patients with advanced NSCLC who were evaluated with a FDG-PET scan prior to first administration of an ICI (nivolumab or pembrolizumab) between 1/2016 and 6/2017. Tumour burden was calculated using the total body Metabolic Tumour Volume (MTV) and the sum of all measurable lesions (SOML) with accordance to the RECIST criteria. This study received IRB approval.
4c3880bb027f159e801041b1021e88e8 Result
A total of 58 patients with histologically proven NSCLC were included. Patients had a median age of 65 years (43-84), 59% were male, 62% had adenocarcinoma and 83% were previously treated with chemotherapy. The median PFS for the entire cohort was 5.7 (1-15.8) months, and the ORR for ICI was 44.8%.
The median MTV was 12.95 (0-236) millimeter³ and was significantly and inversely associated with longer PFS (p=0.036, 95%CI 1-1.015). The median SOML was 88 (13-305) centimetres, and was significantly and inversely associated with a longer PFS and higher ORR (PFS: P=0.004, 95% CI 1.002-1.011, ORR: OR 0.993 p=0.0067(.
Additionally, patients with a SOML under 56 CM (first quartile) had a longer PFS compared to patients with a higher disease volume (Table1).
SOML
(CM)
Median PFS
(months)P value
(compared to 1st quartile)1st quartile 56 12.1 - 2nd quartile 88 5.1 0.017 3rd quartile 115 4.23 0.036 4th quartile 305 3.15 0.01 table 1: some of measurable lesions (in centimeters) and PFS in metastatic NSCLC pateints recieving ICI
8eea62084ca7e541d918e823422bd82e Conclusion
In our study, a high tumour burden in patients with advanced NSCLC treated with ICI was associated with a shorter PFS and a lower ORR. This association warrants further prospective evaluation in order to optimize treatment.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.04-30 - A Potential Effect of Diabetes Mellitus and Metformin Use on Efficacy of Immune Checkpoint Inhibitors (ICI) (ID 14072)
16:45 - 18:00 | Author(s): Nir Peled
- Abstract
Background
Numerous studies have demonstrated metformin use is associated with decreased cancer risk in the general population, as well as improved overall response rate (ORR), progression free survival (PFS) and overall survival (OS) in cancer patients undergoing chemotherapy.Recent in-vitro studies found several new mechanisms which granted metformin the potential to increase cancer patients' response to immune checkpoint inhibitors (ICI).
a9ded1e5ce5d75814730bb4caaf49419 Method
In this study we aim to explore the correlation between the daily use of metformin and benefit from ICI in patients with lung cancer and other solid malignancies.
We retrospectively evaluated all consecutive patients with metastatic solid malignancies treated with ICI therapy in a single institution between February 2015 and June 2017. Patients' clinical data was obtained from electronic medical records. Cox proportional hazards model and chi squared test were used to determine the associations between metformin use and ORR, median PFS (mPFS) and median OS.
4c3880bb027f159e801041b1021e88e8 Result
Of 218 patients included in the analysis (202 NSCLC, 16 non lung cancers), 49 (22.5%) suffered from type 2 diabetes mellitus (T2DM). Of them 33 (15.1%) were treated with metformin and 16 (7.3%) received other, non-metformin therapy for T2DM. Comparison between non-diabetic and diabetic cancer patient groups demonstrated that mPFS was found to be significantly higher in the non-diabetic patients – 6.0 vs. 4.0 months (HR=1.47 [1.03-2.09], p=0.036). ORR was comparable (35.5% vs. 30.6%, p=0.52).
8eea62084ca7e541d918e823422bd82e Conclusion
In the T2DM subgroup - mPFS and HR suggested increased efficacy in the metformin group compared to non-metformin, but the numbers were too small to reach significance 8.0 vs. 3.2 months (HR=0.63 [0.32-1.23], p=0.17). ORR was also numerically higher (36.4% vs. 18.8%, p=0.21).
In both comparisons, no significant differences were found in OS.
This data suggests T2DM might be associated with decreased efficacy of ICI.
While several studies demonstrated that diabetic cancer patients receiving chemotherapy gained much benefit with metformin use, the trend we observed regarding metformin use with ICI therapy was milder and should be further explored in larger prospective cohorts.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-33 - Ex Vivo 2*2*2 Tumor Tissue Explant Culture for Precision Medicine in Immunotherapy and TKI progressors in Lung Cancer (ID 14085)
16:45 - 18:00 | Presenting Author(s): Nir Peled
- Abstract
Background
Lung cancer is one of the leading causes of cancer mortality worldwide. Despite of remarkable progress made in the lung cancer therapy, an unmet need is there in tailoring the appropriate patient specific therapy due to a variety of treatment options. Our aim was to develop a high-throughput drug screening method of tumor ex-vivo analysis (TEVA) which can predict patient-specific drug response and thus can be used for personalized cancer treatment.
a9ded1e5ce5d75814730bb4caaf49419 Method
Freshly operated tumor tissue samples from non small cell lung carcinoma (NSCLC) patients were received from Soroka Medical Center, Israel and implanted in NSG mice to form PDXs. We developed a method that enabled us to cut the PDXs into 2*2*2mm tissue explants and then treated with clinically relevant drugs (Genomics/proteomics suggested) in 48 well plates for 24 hours. TMA blocks were prepared and IHC was performed. Parameters, such as Ki67, TUNEL and respective signalling molecules (pMAPK, pPRAS40) were chosen to predict the drug response ex vivo and a score was given to each drug based upon those parameters.
4c3880bb027f159e801041b1021e88e8 Result
8 NSCLC patients have been enrolled so far. Implantation rate was 75%. 15 drugs combinations have been developed to test the most common conditions, i.e. progression on EGFR TKIs, progression on immunotherapy and progression on chemotherapy. Preliminary results indicate a potential role for MET blockade in immune-resistant as well as in EGFR progressors. We also observed that the TEVA score correlated with the genomics/proteomics based drug results.
8eea62084ca7e541d918e823422bd82e Conclusion
Overall, this low cost, fast, relatively simple and efficient method can bypass the necessity of drug validation in mice and can be used for multiple drug screening to select a precise patient specific drug. The method is feasible and expose ways to overcome acquired resistant to novel drugs.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-02 - Osimertinib for EGFR-Positive Advanced NSCLC with Brain Metastases: Preliminary Analysis of an Open-Label, Two-Arm, Phase 2 Study (ID 13020)
16:45 - 18:00 | Presenting Author(s): Nir Peled
- Abstract
Background
Osimertinib is an EGFR tyrosine-kinase inhibitor (TKI) selective for both EGFR TKI sensitizing and Thr790Met resistance mutations. While intracranial activity of osimertinib was observed in EGFR-mutant NSCLC in larger trials, a study focusing on patients with brain metastases was not reported yet.
a9ded1e5ce5d75814730bb4caaf49419 Method
This phase 2, open-label, two-arm study enrolled patients with EGFR-mutant, advanced NSCLC and at least one asymptomatic brain metastasis. Treatment-naïve (arm A) and Thr790Met-positive patients who progressed on EGFR-TKI therapy (arm B) received osimertinib 80 mg QD. Dose escalation (160 mg QD) was performed in cases of intracranial progression without symptomatic systemic progression. The primary endpoint was intracranial metastasis response. The trial is ongoing (NCT02736513 at ClinicalTrials.gov) and here we present a preliminary analysis.
4c3880bb027f159e801041b1021e88e8 Result
Between May 31, 2016, and November 30, 2017 (data cutoff), 20 patients started osimertinib (arm A=15, arm B=5). Median duration of follow-up was 43 weeks. Intracranial response was achieved in 11 (73%; 95% CI 45%-92%) of 15 arm A and in four (80%; 95% CI 28%-99%) of five arm B patients. Dose escalation was performed in four cases (arm A=2, arm B=2), with one continuous response (25%, 95% CI 5%-70%). Ten of 15 patients (67%) in arm A and one of five patients (20%) in arm B continue responding to osimertinib 80 mg at data cutoff. Median intracranial PFS (80 mg) was not available for arm A (95% CI 232 days–NA), and was 510 days in arm B (95% CI 161–not available). Toxic effects were similar to previous reported data.
8eea62084ca7e541d918e823422bd82e Conclusion
Osimertinib shows equal intracranial and systemic activity with minor side-effects in EGFR-mutant NSCLC as first-line, as well as in previously treated Thr790Met-positive patients. It might therefore be a reasonable treatment for these patient populations and defer brain radiotherapy.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 961)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.12-14 - Stereotactic Radiosurgery for Brain Metastases in Small Cell Lung Cancer. (ID 13726)
16:45 - 18:00 | Author(s): Nir Peled
- Abstract
Background
Prophylactic cranial irradiation (PCI) omission in favor of brain magnetic resonance imaging (MRI) staging and surveillance in the management of small cell lung cancer (SCLC) is controversial yet accepted by some centers policy. The latter strategy implies stereotactic radiosurgery (SRS) treatment (Tx) for limited brain metastases (BM). Data regarding SRS efficacy in this setting is limited.
a9ded1e5ce5d75814730bb4caaf49419 Method
Ten consecutive SCLC patients (pts) with BM treated with SRS at Davidoff Cancer center between Aug 2012 and July 2017 were identified through the institutional database; pts receiving PCI or whole brain radiotherapy (WBRT) as a primary intervention were excluded. Brain MRI images were reviewed by neuro-radiology specialist. Intracranial objective response rate (IORR), progression-free survival (IPFS), intracranial failure patterns, overall survival (OS) and time-to-WBRT/death whichever occurred first (TTWD) were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
Baseline pt characteristics are presented in the Table. SRS dose ranged from 16 Gy to 22.5 Gy. IORR comprised 57% by RECIST 1.1 and 60% by mRECIST 1.1. Intracranial progression developed in 8 pts; median IPFS was 3.9 mo (95% CI, 1.7-7.2). In-site, off-site and combined pattern of intracranial failure was seen in 0, 6, and 2 pts, respectively; median number of new BM per disease course was 2 (range, 1-11). Nine additional rounds of SRS were delivered in 6 pts (median number of lesion irradiated per round-1, range, 1-5). WBRT was ultimately administered in 3 pts; 4 pts died. Median TTWD comprised 23.2 mo (95% CI, 1.9-26.8). Median OS since SRS administration was 23.2 mo (95% CI, 4.2-26.8).
8eea62084ca7e541d918e823422bd82e Conclusion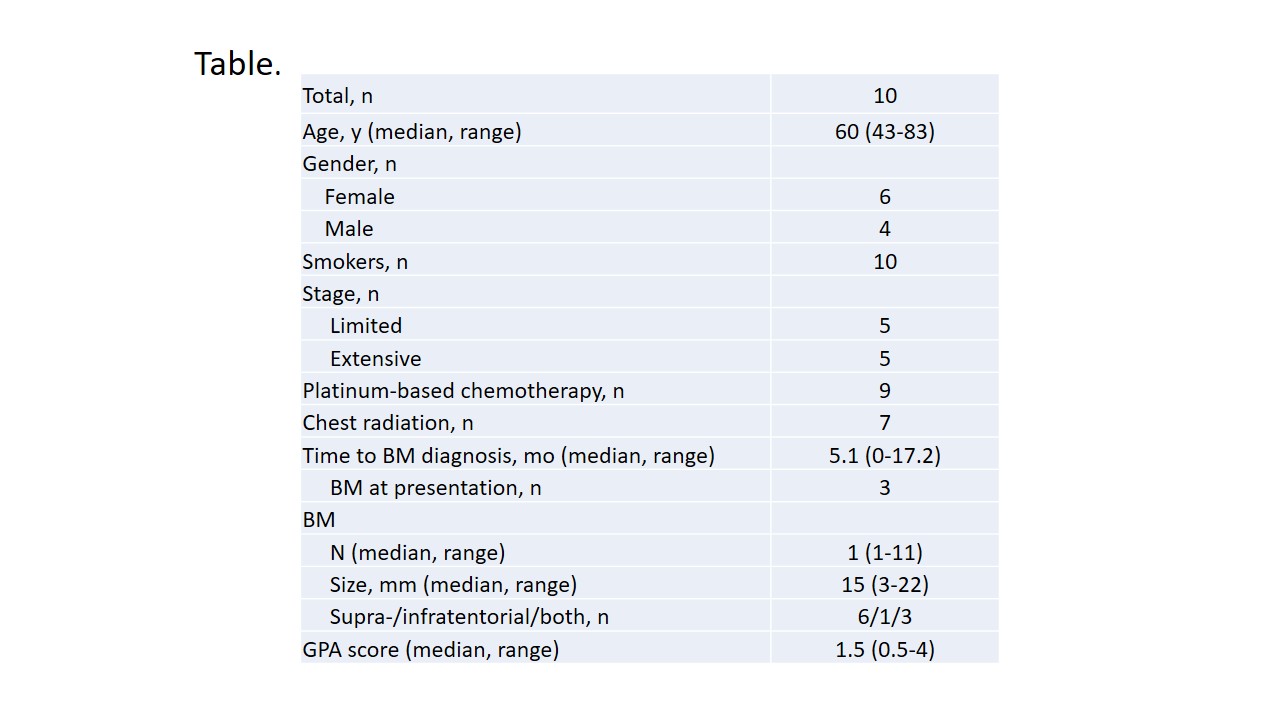
MRI surveillance combined with multiple rounds of SRS in case of limited BM development might provide reasonable alternative to PCI or therapeutic WBRT in SCLC. SRS in SCLC warrants prospective evaluation.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-28 - The Clinical Impact of Comprehensive cfDNA Genomic Testing in Lung Cancer (ID 13878)
12:00 - 13:30 | Author(s): Nir Peled
- Abstract
Background
Next-generation sequencing (NGS) of cell-free circulating tumor DNA (cfDNA) enables a non-invasive option for comprehensive genomic analysis of non-small cell lung cancer (NSCLC) patients. Although plasma-detected genomic alterations have been shown to predict targeted therapy response, evidence of durability of response is lacking or limited to small cohorts as is the impact of cfDNA NGS results on clinical decision making.
a9ded1e5ce5d75814730bb4caaf49419 Method
In this retrospective study, data was collected on stage IIIB/IV NSCLC patients between the years 2014-2017 in Israel. We utilized cfDNA NGS (Guardant360) which covers the seven genes targetable with FDA-approved therapies in NSCLC.
4c3880bb027f159e801041b1021e88e8 Result
116 consecutively NSCLC patients were tested, 41.4% (48/116) before 1st line therapy (Group A), 34.5% (40/116) upon progression on chemotherapy or immunotherapy (Group B1) and 24.1% (28/116) upon progression on EGFR TKIs (Group B2). Targetable genomic alterations were found in 65% of group A (15/48), 53% in group B1 (21/40) and 71% in group B2 (20/28). Treatment decision was changed to targeted therapy based on cfDNA NGS analysis in 23% (11/48), 25% (10/40) and 32% (9/28), respectively (total cohort 26%; 30/116). Response assessment (RECIST) showed complete response in 4% (1/28), partial response in 39% (11/28), stable disease in 32% (9/28) and progressive disease in 25% (7/28). Total objective response rate (ORR) was 43% and disease control rate was 75% for 5 months treatment duration.
8eea62084ca7e541d918e823422bd82e Conclusion
Comprehensive cfDNA testing impacted clinical decisions in 23% of naïve patients, 25% in patients who progressed on chemotherapy and 32% in EGFR TKI progressors. Median treatment duration was 5 months. This retrospective study extends previous reports by showing that responses based on cfDNA are durable and change treatment decisions at initial presentation and at progression.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.03 - Biology (Not CME Accredited Session) (ID 969)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.03-15 - Lung Cancer Regulation of Glucose Metabolic Stress Response (ID 13850)
12:00 - 13:30 | Author(s): Nir Peled
- Abstract
Background
Cancer cells have altered metabolism in order to accommodate for the increased metabolic needs required for oncogenic transformation, proliferation, development and survival. The metabolic profile observed in cancer cells often includes increased consumption of glucose and glutamine, increased glycolysis, changes in the use of metabolic enzyme isoforms, and increased secretion of lactate. In order to better understand this hallmark of cancer, we studied the regulation of pathways by microRNAs (miRNA) expression profile under normal or deprived glucose condition in different lung cancer cell lines.
a9ded1e5ce5d75814730bb4caaf49419 Method
Two different NSCLC adenocarcinoma cell lines were tested in the study, A549, which is highly glycolytic (HG) and H358, which is low glycolytic (LG). Sequencing was performed using Illumina TruSeq RNA library preparation kit v2 with Illumina HiSeq 2500 sequencer using single-end 50bp protocol. Cell were analysed in two different conditions, under 24h of normal glucose concentration and under 24h glucose starvation. Samples were prepared in duplicates from the initial step of cell culturing summarizing to a total of eight samples. Differential expression analysis was performed using EdgeR algorithm with an adjusted p-value >0.05 and a log2 fold > 1.5.
4c3880bb027f159e801041b1021e88e8 Result
An opposite regulation profile was observed for miRNA of cells that are highly glycolytic (HG), A549, in comparison with low glycolytic, H358 cells. The formers presented 42 downregulated miRNAs from which 39 miRNAs were unique. Only 2 upregulated miRNAs, miR324 and miR616, were found significant in this cohort but they are also upregulated in the latter group. In comparison, from the 20 upregulated miRNAs found in high-glycolygic group from which 18 were unique to lower glycolytic cell line. Lower glycolytic cell lines show 4 downregulated miRNAs from which only one miRNA, miR205 was significantly uniquely downregulated, moreover, MIR205HG, which is miR205 host gene, is also found significantly downregulated, suggesting the strong involvement of miR205 in glucose metabolic stress response.
8eea62084ca7e541d918e823422bd82e Conclusion
Under glucose metabolic stress, miR205 and MIR205HG are uniquely downregulated in low glycolytic NSCLC adenocarcinoma cell line.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.04 - Immunooncology (Not CME Accredited Session) (ID 970)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.04-11 - The Influence of Circulating Tumor DNA Analysis on Response to Immunotherapy in Non-Small Cell Lung Cancer (NSCLC) (ID 13810)
12:00 - 13:30 | Presenting Author(s): Nir Peled
- Abstract
Background
In advanced NSCLC, immunotherapy has demonstrated good response rates with some responses being remarkably durable, however emerging biomarkers predictive of response (or lack thereof) include PD-L1 expression, tumor mutation burden (TMB), genomic alterations in EGFR/ALK/ROS1 and KRAS/TP53/STK11 mutations, all competing for limited tissue biopsy samples. Therefore, we investigated whether these biomarkers can be detected from a non-invasive plasma sample. In particular, challenges to assessment of TMB with cell-free DNA next-generation sequencing (NGS) include the limited size of liquid biopsy gene panels and the fact that low shedding of tumor DNA into circulation may fail to detect hypermutated tumors. We previously reported, in a modest 27 advanced NSCLC cohort, that TMB can be ascertained with a 73-gene cfDNA NGS panel that adjusts for the degree of tumor shedding, and here investigate whether detection of TMB and the above genomic mutations can be used to predict immune checkpoint inhibitor response in a larger cohort.
a9ded1e5ce5d75814730bb4caaf49419 Method
In this retrospective study, data were collected on NSCLC patients treated in multiple medical centers in Israel between 2014 and 2017. We used NGS on cell-free circulating tumor DNA (ctDNA) to evaluate whether mutational burden and specific genomic alterations influence the response to immunotherapy in these patients.
4c3880bb027f159e801041b1021e88e8 Result
Overall, 336 NSCLC patients underwent NGS on ctDNA. Of these 336 patients, 192 (57%) were females and 144 (43%) were males. The average age (range) was 64 (23-103) years. Clinical treatment information is currently available for 117 patients, of whom 50 (43%) received immune check-point inhibitors. Rates of stable disease, partial and complete responses (RECIST criteria), as well as progression-free survival and overall survival will be reported. In addition, to unravel the genomic determinants of response to immunotherapy we will use the blood-derived ctDNA to understand if hypermutated ctDNA is a predictive biomarker of response to immunotherapy.
8eea62084ca7e541d918e823422bd82e Conclusion
ctDNA collection was feasible in 336 patients. A prediction model to associate the ctDNA signature with response to immunotherapy based on plasma will be presented.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P3.04-28 - Can Ipilimumab Restore Immune Response in Advanced NSCLC After Progression on Anti PD1/PDL1 Agents? (ID 13775)
12:00 - 13:30 | Author(s): Nir Peled
- Abstract
Background
Anti PD1/PDL1 agents play a crucial part in the treatment of NSCLC demonstrating improved overall response rate (ORR) and overall survival. While anti CTLA4 alone did not show significant single agent activity, a phase 1 study evaluating the combination with anti PD1 suggests improved ORR.
Evidence is scarce regarding subsequent treatment with immune checkpoint inhibitors (ICI) after progression on anti PD1/PDL1. A recent study in melanoma patients reported lack of benefit from the addition of Ipilimumab after progression on anti PD1.
a9ded1e5ce5d75814730bb4caaf49419 Method
All off-label Ipilimumab administrations in a single tertiary center were retrieved through the institutional review board records. 21 patients with advanced NSCLC were allocated during 2017. Clinical data were retrieved retrospectively. Disease control was define as partial response (PR) or stable disease (SD).
4c3880bb027f159e801041b1021e88e8 Result
Table 1 - Patients’ characteristics N-15 (%) Sex M-15, F-0 Median age (range) 67 (53-87)
Histology Adenocarcinoma
12 (80%)
Squamous
1 (6.7%)
Adenosquamous 1 (6.7%)
NSCLC-NOS 1 (6.7%) PD-L1 expression >50%
2 (13.3%)
1-50%
4 (26.7%)
<1%
2 (13.3%)
Unknown 7 (46.7%) Line of Tx with anti PD1/PDL1 1st
2 (13.3%)
2nd 12 (80%)
3rd 1 (6.7%) Best response to anti PD1/PDL1 PR 5 (33.3%)
SD
2 (13.3%)
PD 8 (53.4%) Median time to progression on anti PD1 (range) 9 mon. (6-17) Of 21 patients who were treated with a combination of anti PD1 agent and Ipilimumab, in 15 Ipilimumab was initiated after confirmed progression on anti PD1/PDL1 alone. Patients' characteristics are described in table 1.
The overall disease control rate was 33.3% (n-5); 3 patients with PR and 2 patients with SD, 3 of them had previously responded to anti PD1. Immune related AE rate was 40% (n-6); 2 patients had grade 3 AE and one patient died of pneumonitis. While the median time to progression was 2 months, 4 of the 5 responding patients are still stable after median 4 months follow up (range 1-15).
8eea62084ca7e541d918e823422bd82e Conclusion
This small retrospective cohort suggests Ipilimumab might re-boost immune response in patients with advanced NSCLC progressing on anti PD1 therapy, while delaying exposure to the higher rates of AE associated with upfront combination therapy. This strategy should be explored prospectively.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.11 - Screening and Early Detection (Not CME Accredited Session) (ID 977)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.11-22 - The Path to National Lung Cancer Screening Program in Israel (ID 11917)
12:00 - 13:30 | Author(s): Nir Peled
- Abstract
Background
2500 people are diagnosed every year with lung cancer and 2000 die from the disease, the leading cause of death in Israel. The prevalence of smoking is 22.5% above 21 years of age, resulting in about 1.2 million smokers overall. The USPSTF found adequate evidence that annual screening for lung cancer with LDCT in a defined population of high-risk persons can prevent a substantial number of lung cancer–related deaths, therefore the Israeli Lung Cancer foundation (ILCF) decided to advocate for including LC screening program in Israel's medical services.
a9ded1e5ce5d75814730bb4caaf49419 Method
The medical services "health basket" in Israel encompasses the entire range of services, drugs, medical equipment and devices that the insured public receives. Every end of year, a health basket committee is appointed to review and approve the new services to be included in the following year health basket. The services are reviewed for their health benefit and then are chosen to fit a limited budget.
ILCF have filled twice for the years of 2017 and 2018 to have a national LDCT LC screening program in Israel.
4c3880bb027f159e801041b1021e88e8 Result
LC Screening program was filled by ILCF to the 2017 Health basket committee. The committee decided that there is not enough supporting evidence and did not rank the program high enough to be included in the funding debate. Nevertheless, because the issue was raised, the ministry of health decided to gather a special committee to evaluate LC Screening program. The committee decided that there is enough evidence and recommended to have the program be part of the health system in Israel.
For 2018-year, LC Screening program was filled by ILCF and the ministry of health LC Screening committee. This time after an additional appeal the program was ranked to enter the funding debate. Unfortunately, it was excluded from the 2018 health basket. As a result, ILCF appealed to the Israeli high court of justice against the health basket committee claiming that the decision was unreasonable. The appeal is being currently discussed.
8eea62084ca7e541d918e823422bd82e Conclusion
Change takes a lot of effort and collaboration. Unfortunately, lung cancer screening is not an industrial initiative, thus does not have strong lobbying power. Therefore, it is the duty of patient advocates and leading oncologists, radiologists and pulmonologists to raise the flag. Here we present a model of collaboration between a patient organization and leading oncologists and radiologists to incorporate LC Screening program in Israel.
6f8b794f3246b0c1e1780bb4d4d5dc53


