Virtual Library
Start Your Search
David Raymond Baldwin
Author of
-
+
MS04 - Joint GLCC/IASLC Session: Exploring Hot Topics for Advocates (ID 783)
- Event: WCLC 2018
- Type: Mini Symposium
- Track: Advocacy
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 206 BD
-
+
MS04.02 - How Is Lung Cancer Screening Evolving to Be More Efficient and Effective? (ID 11416)
10:45 - 11:00 | Presenting Author(s): David Raymond Baldwin
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 944)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.12-12 - A Systematic Review and Meta-Analysis of Early and Late Survival Following Anti-Cancer Therapies for Small Cell Lung Cancer (ID 13476)
16:45 - 18:00 | Author(s): David Raymond Baldwin
- Abstract
Background
Treatments for small cell lung cancer (SCLC) have not changed significantly in contrast to non-small cell where it is more individually tailored. Current guidelines generally have a one size fits all approach to chemotherapy. We conducted the largest systematic review and meta-analysis in SCLC to evaluate early and median survival by different study factors.
a9ded1e5ce5d75814730bb4caaf49419 Method
We searched EMBASE and MEDLINE for randomized controlled trials and observational cohort studies which reported survival following platinum doublet chemotherapy for SCLC. We calculated overall survival at 30 and 90 days along with the median survival.
4c3880bb027f159e801041b1021e88e8 Result
We identified 10,487 titles, 161 were included. Cisplatin + etoposide (n=87 (49.4%)), carboplatin + etoposide (n=36 (20.5%)) and cisplatin + irinotecan (n=23 (13.1%)) were predominantly reported. The commonly reported cause of death within 30 days was neutropaenic sepsis (n=27), disease progression (n=11) and cardiovascular (n=8). Across both stages 30-day survival was 98% (95% CI 98-99%) whilst 90-day was 95% (95% CI 94-96%). Thirty and 90-day survival showed similar patterns to study factors as median survival (summarised in Table 1).
Limited stage median survival was 18.1 months (95% CI 17.0-19.1). Studies that administered thoracic radiotherapy and PCI had better survival than those that did not. Studies giving carboplatin + etoposide or included poorer PS (0-3) individuals had inferior survival. PCI timing did not show survival differences.
Extensive stage median survival was 9.6 months (95% CI 8.9-10.3). This was augmented in studies that gave irinotecan + cisplatin and were conducted in Asia. There were no survival differences by cisplatin/carboplatin or median participant age.
8eea62084ca7e541d918e823422bd82e Conclusion
Neutropenic sepsis accounts for the majority of 30-day deaths and was mostly reported with cisplatin + etoposide. Our findings broadly support guideline recommendations but suggest certain sub-populations e.g. Asian individuals, benefit from targeted treatment with irinotecan + cisplatin. Age should be re-considered as a treatment-deciding factor in extensive stage.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.11 - Screening and Early Detection (Not CME Accredited Session) (ID 960)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.11-28 - A Focus Group and Interview Study to Explore the Information-Needs of Lung Cancer Screening Participants (ID 12753)
16:45 - 18:00 | Author(s): David Raymond Baldwin
- Abstract
Background
Lung cancer screening (LCS) by low-dose CT has been shown to improve mortality, but individuals must consider the potential benefits and harms before making an informed decision about taking part. In the US a shared decision-making process is mandated to qualify for LCS reimbursement. However, screening eligible individuals’ (SEI) specific views of these harms, and their preferences for accessing this information, are not well described.
a9ded1e5ce5d75814730bb4caaf49419 Method
16 semi-structured interviews were carried out with general practitioners, public health consultants, respiratory physicians and lung cancer nurse specialists. 35 SEI participated in seven focus groups, which were divided into current vs. former smokers and lower vs. higher educational backgrounds. Interviews and focus groups were audio-recorded and transcribed. Data were coded inductively and analysed using the framework method.
4c3880bb027f159e801041b1021e88e8 Result
Lung cancer was generally perceived as an incurable condition, and smokers appeared to be particularly fatalistic. Despite this, a ‘belief in screening’ emerged from the interviews and focus groups. Participants’ appetite for information varied; with many expressing a ‘right’ to be given all the information, while others cautioned against too much information, and HCPs also acknowledged this dichotomy. Of the harms of screening, false positives and false negatives generated the most concern, though for most participants, even these were unlikely to deter them from screening. Participants were aware of the harms of smoking though many current smokers perceived other factors as more detrimental to their health than smoking. Regarding smoking cessation advice at screening, most focus group and interview participants preferred an emphasis on the benefits of quitting, and for advice to be delivered in a positive and empowering manner.
8eea62084ca7e541d918e823422bd82e Conclusion
These findings can be used to directly inform the way in which information is presented to SEI in order to aid the shared decision-making process, motivate smoking cessation and minimise barriers to uptake of LCS.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.11-29 - Impact of an Information-Film to Promote Informed Decision-Making in Individuals Taking Part in a Lung Cancer Screening Demonstration Pilot (ID 12757)
16:45 - 18:00 | Author(s): David Raymond Baldwin
- Abstract
Background
Lung cancer screening by Low Dose CT (LDCT) is underway in the United States, where a shared decision-making process is mandated for insurer funding. The potential harms of screening are complex and difficult to communicate. Participants do not always read written materials and audio-visual aids have been shown to improve informed decision-making in other areas of medicine. There are limited studies on the use of decision aids in lung cancer screening.
a9ded1e5ce5d75814730bb4caaf49419 Method
A five-minute information-film was made to explain the benefits and risks of lung cancer screening. Qualitative research informed the content and format to make it accessible for individuals of varying demographic and educational backgrounds. A sub-sample of participants (n=229) from a lung cancer screening pilot were randomised to watch the film and read a written information booklet (Group A) or read the booklet alone (Group B). Objective and subjective knowledge of the risks and benefits of screening were assessed before and after the intervention, and again after discussion with a heath care professional, when decisional conflict and satisfaction were also assessed.
4c3880bb027f159e801041b1021e88e8 Result
120 and 109 participants were randomised to groups A and B respectively. There was a statistically significant improvement in subjective and objective knowledge scores post-administration of the intervention in both groups (p<0.001). In a multivariate analysis adjusted for educational level and baseline knowledge score, post interventional scores were independently associated with film group (p=0.036). There were no group differences in decisional conflict or satisfaction, or in subsequent attendance for LDCT. Participants’ views on the acceptability of the film are presented in table 1.
Table 1: Feedback and acceptability of information film
8eea62084ca7e541d918e823422bd82e Conclusion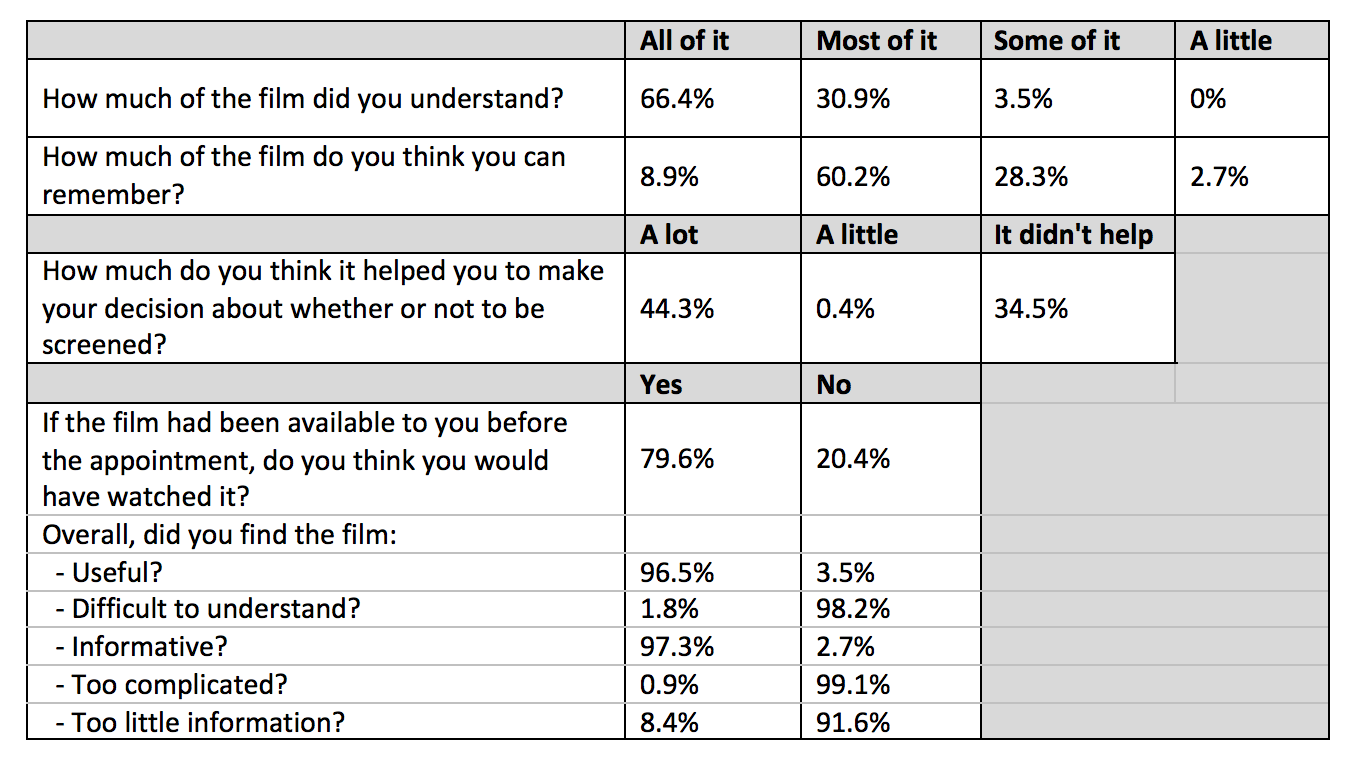
The information-film was well-accepted and increased knowledge scores more than a written booklet alone, without raising decisional conflict or reducing attendance for LDCT.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.16 - Treatment of Early Stage/Localized Disease (Not CME Accredited Session) (ID 965)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.16-16 - SABRTOOTH: A Fasibility Study of SABR Versus Surgery in Patients with Peripheral Stage I NSCLC Considered to be at Higher Risk for Surgery. (ID 13679)
16:45 - 18:00 | Author(s): David Raymond Baldwin
- Abstract
Background
Stage I NSCLC is curable by surgery and Stereotactic Ablative Radiotherapy (SABR). Many patients have co-morbidities that place them at higher risk of surgical complications. For such patients it is unknown whether the potential benefits of surgery are outweighed by the risks since published randomised trials comparing surgery with SABR have been underpowered. The SABRTooth study was designed to determine the feasibility of randomising patients between the two treatments and thus performing a larger RCT.
a9ded1e5ce5d75814730bb4caaf49419 Method
Four thoracic oncology centres and a referral site participated. Patients with peripheral (>2cm from the main airways) stage T1-T2bN0M0 NSCLC were considered for study entry. Patients at higher risk were identified using several criteria including Thoracoscore and the Nottingham Risk Score and confirmed by multidisciplinary team consensus.
Eligible patients were approached by a respiratory physician and research nurse, consented and randomised (1:1) before consulting a surgeon or oncologist. Surgery was preferably by lobectomy with lymph node sampling/resection although sub-lobar resection was permitted. SABR was delivered as per the UK SABR guidelines.
An average recruitment rate of 3 patients/month from the 5 centres over a formal monitoring period was set to prove feasibility of a larger RCT. Meetings with the trial sites and patient representatives were held through-out to improve recruitment. Qualitative research was embedded into the study with interviews for patients who declined participation or randomised treatment.
4c3880bb027f159e801041b1021e88e8 Result
Between July 2015-January 2017 318 patients were assessed for eligibility of which 106 were initially considered eligible. 84 patients were approached for the study and 24 (29%) were randomised (10 surgery, 14 SABR); a mean recruitment rate of 1.7 per month. The median age was 75 (range 54-88). The main reason for declining the study was patient preference with 29% preferring surgery and 42% SABR. Overall 9/24 (38%) did not receive their randomised treatment. Of 7 patients randomised to surgery, 6 received SABR, 1 radical radiotherapy and of 2 patients randomised to SABR, 1 received radical radiotherapy, 1 was lost to follow-up.
8eea62084ca7e541d918e823422bd82e Conclusion
Despite recruiting at higher rate/centre than previous SABR versus surgery trials, the SABRTooth study failed to meet its recruitment target and the majority of patients randomised to surgery subsequently underwent SABR. Therefore, conducting a large RCT in the UK was shown not to be feasible. However, establishing which patients should have surgery or SABR for early stage NSCLC remains a critical question and alternative study designs are being developed to provide an answer for patients and clinicians.
6f8b794f3246b0c1e1780bb4d4d5dc53


