Virtual Library
Start Your Search
Qing Zhou
Author of
-
+
JCSE01 - Perspectives for Lung Cancer Early Detection (ID 779)
- Event: WCLC 2018
- Type: Joint IASLC/CSCO/CAALC Session
- Track: Screening and Early Detection
- Presentations: 2
- Moderators:
- Coordinates: 9/23/2018, 07:30 - 11:15, Room 202 BD
-
+
JCSE01.09 - Cluster Trial: Ph2 Biomarker-Integrated Study of Single Agent Alpelisib, Capmatinib, Ceritinib and Binimetinib in advNSCLC (ID 11678)
10:15 - 10:25 | Presenting Author(s): Qing Zhou
- Abstract
- Presentation
Background
Several genetically altered signaling pathways have been profiled in NSCLC, enabling advanced management of NSCLC using targeted therapies. This study investigated the therapeutic spectrum of NSCLC with uncommon molecular alterations by allocating patients to treatment arms based on molecular aberrations; targeted therapies alpelisib (PI3Kαi), capmatinib (METi), ceritinib (ALKi), and binimetinib (MEKi) were evaluated.The study was based on the umbrella design. Key objectives: investigate feasibility of using one trial for different agents based on biomarker-integrated analysis, assess anti-tumor activity, characterize safety, tolerability and PK profiles of individual agents. Key eligibility criteria: age ≥18 years; ECOG PS ≤2; failed prior treatment/unsuitable for chemotherapy. Documentation of locally determined molecular alterations before treatment allocation was required (alpelisib, 350 mg QD: PIK3CA mutation/amplification; capmatinib, 400 mg BID (tablet): MET IHC overexpression/amplification; ceritinib, 750 mg QD: ALK or ROS1 rearrangement; binimetinib, 45 mg BID: KRAS, NRAS or BRAF mutation).Sixty-six patients with advNSCLC were enrolled (median age 58 years; 65.2% male: alpelisib, n=2; capmatinib, n=16; ceritinib, n=26; binimetinib, n=22). As of Feb 28, 2018, 10 patients in ceritinib and 2 in binimetinib arms were ongoing. Twenty-four patients had confirmed partial responses (36.4%): alpelisib, 0%; capmatinib, 18.8%; ceritinib, 73.1%; binimetinib, 9.1% (Figure). Longest mPFS (14.4 months) was in ceritinib arm. Among the most common treatment-related AEs: alpelisib: malaise, hyperglycemia, dysgeusia; capmatinib: nausea, anemia, peripheral edema, decreased appetite; ceritinib: diarrhea, vomiting, ALT/AST elevation; binimetinib: mouth ulceration, AST, blood CPK increased, rash. Most AEs were grade 1/2.
Objective responses/tumor shrinkage were observed in the study; highest ORR and mPFS were observed with ceritinib, although patient numbers differed between arms. All treatments were well tolerated; no new safety signals were observed. This study demonstrated the feasibility of an umbrella trial and importance of precision medicine in the management of advNSCLC with uncommon molecular alterations.
a9ded1e5ce5d75814730bb4caaf49419Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
JCSE01.18 - A Multicenter Survey of One Year Survival Among Chinese Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer (CTONG1506) (ID 14707)
11:15 - 11:15 | Presenting Author(s): Qing Zhou
- Abstract
Background
Previous results of CTONG1506 study showed that gene aberration test rate was increasing in Chinese NSCLC patients and first-line treatment was standardized accordingly. This survey further described one year survival of patients with different gene aberration status and under different first-line treatments.CTONG1506 was a two-year series cross-sectional study. Patients with advanced nonsquamous NSCLC who were admitted from August 2015 to March 2016 and who received first-line anti-cancer treatment at one of 12 tertiary hospitals across China were included. Data extracted from medical charts were entered into medical record abstraction forms, which were collated for analysis. Survival information was collected one year after patients were admitted to hospital. One year survival rate and its 95% confidence interval were analysed by Kaplan-Meier method.
A total of 707 patients were analysed, with mean age of 57 years and 56.7% were male. Among the 487 patients who had survival data, 192 were EGFR- mutation positive (86 mutated in exon 19 [one year survival rate 0.90, 95% CI: 0.81-0.94] and 88 mutated in exon 21 [one year survival rate 0.84, 95% CI: 0.75-0.90]), 27 patients were ALK positive and 164 patients were EGFR and ALK wild type. Most EGFRmutation positive patients (128/192) received tyrosine kinase inhibitors (TKIs) as first-line treatment and most EGFR wild type patients (155/175) received first-line chemotherapy (Chemo). Pemetrexed was the most common non-platinum chemotherapy-backbone agent (120/155) in platinum doublet regimens. One year survival rates are shown in the table.
This national-wide real world study of tertiary hospitals in China revealed that a majority of (>75%) advanced nonsquamous NSCLC patients survived more than one year and was comparable to well-controlled clinical trial results, indicating survival benefits by gene aberration status guided standard of care. This result may be further validated by our on-going two-year survey.
a9ded1e5ce5d75814730bb4caaf49419
-
+
MA02 - Improving Outcomes for Patients with Lung Cancer (ID 895)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 201 BD
-
+
MA02.03 - ASTRIS: A Real World Treatment Study of Osimertinib in Patients with EGFR T790M-Positive NSCLC (ID 12972)
10:40 - 10:45 | Author(s): Qing Zhou
- Abstract
- Presentation
Background
Osimertinib is a third-generation, CNS-active EGFR-TKI that potently and selectively inhibits both EGFR-TKI sensitizing and EGFR T790M resistance mutations. We report results from a second planned protocol, optimal interim analysis of the ongoing ASTRIS study (NCT02474355).
a9ded1e5ce5d75814730bb4caaf49419 Method
Eligible patients receive osimertinib 80 mg once daily. Inclusion criteria: stage IIIB/IV T790M-positive non-small cell lung cancer (NSCLC); T790M status confirmed locally by validated test, not restricted by sample type; prior EGFR-TKI therapy received; WHO performance status (PS) 0−2; acceptable organ and bone marrow function and no history of interstitial lung disease (ILD) or QTc prolongation. Asymptomatic, stable CNS metastases are permitted. The primary efficacy outcome is overall survival (OS).
4c3880bb027f159e801041b1021e88e8 Result
From Sept 18, 2015, first patient in, to Oct 20 2017 data cut-off (DCO), 3014 patients were enrolled across 16 countries and received ≥1 dose of osimertinib (full analysis set [FAS]): median follow-up 7.9 months (range <1−24), median age 62 yrs (27–92), 64% female, 69% Asian, 30% White, 11% WHO PS 2, 45% prior chemotherapy, 34% prior radiotherapy. All patients had T790M-positive status, identified from tissue in 1610 patients (53%), plasma ctDNA in 1241 patients (41%) and from other sources in 162 patients (5%). At DCO, 1276 patients (42%) had discontinued treatment (1738 [58%] ongoing); median duration of exposure 7.4 months (<1–25); 1289 patients (43%) had a progression-free survival (PFS) event, 1276 (42%) had a time to treatment discontinuation (TTD) event, and 593 (20%) had died. In patients evaluable for response, the investigator-assessed clinical response rate was 56.6% (1625/2872; 95% confidence interval [CI] 54.7, 58.4). In the FAS, estimated median PFS was 11.0 months (95% CI 10.6, 11.1), median TTD was 12.6 months (95% CI 12.2, 13.7), and median OS was not reached (OS at 12 months was 75.8% (95% CI 73.7, 77.8). Adverse events (AEs) leading to dose modification and treatment discontinuation were reported in 321 patients (11%) and 147 patients (5%), respectively. Serious AEs were reported in 505 patients (17%). ILD/pneumonitis-like events were reported in 41 patients (1%), and QTc prolongation in 48 patients (2%).
8eea62084ca7e541d918e823422bd82e Conclusion
ASTRIS, the largest reported study of osimertinib in T790M-positive NSCLC, demonstrates clinical activity similar to that observed in the osimertinib clinical trial program with no new safety signals.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15 - Colliding Approaches - EGFR and Immunotherapy (ID 916)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 107
-
+
MA15.01 - Strong PD-L1 Expression Predicts Poor Response and de Novo Resistance to EGFR TKIs Among Non-Small Cell Lung Cancer Patients with EGFR Mutation (ID 12920)
13:30 - 13:35 | Author(s): Qing Zhou
- Abstract
- Presentation
Background
This study evaluated whether tumor expression of programmed death-ligand 1 (PD-L1) predicted the response of EGFR-mutated non-small cell lung cancer (NSCLC) to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs).
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively evaluated patients who received EGFR-TKIs for advanced NSCLC between April 2016 and September 2017 at the Guangdong Lung Cancer Institute. None of them were enrolled in clinical studies, and their EGFR and PD-L1 statuses were simultaneously evaluated.
4c3880bb027f159e801041b1021e88e8 Result
Among the 101 eligible patients, strong PD-L1 expression significantly decreased the objective response rate (ORR) compared with those with weak or negative PD-L1 expression (35.7% vs 63.2% vs 67.3%, P=0.002) as well as shortened progression-free survival (PFS, 3.8months vs 6.0months vs 9.5months, P<0.001), regardless of EGFR mutation types (19del or L858R). Furthermore, positive PD-L1 expression was predominantly observed among patients with de novo resistance rather than acquired resistance to EGFR-TKIs (66.7% vs 30.2%, P=0.009). Notably, patients with de novo resistance had a high proportion of dual positive for PD-L1 and CD8 (46.7%, 7/15). Finally, one patient with de novo resistance to EGFR-TKIs and dual positivity for PD-L1 and CD8 experienced a favorable response to anti-PD-1 therapy.
8eea62084ca7e541d918e823422bd82e Conclusion
This study uncovered the adverse impact of PD-L1 expression on the efficacy of EGFR- TKIs, especially in those with de novo resistance NSCLC, which inclined to reshape an inflamed immune phenotype of dual positive for PD-L1 and CD8 and showed potential therapeutic sensitivity to PD-1 blockade.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.06 - Circulating Tumor DNA Portrays the Resistance Landscape to a Novel Third Generation EGFR Inhibitor, AC0010 (ID 13641)
14:05 - 14:10 | Author(s): Qing Zhou
- Abstract
- Presentation
Background
In a Phase I/II dose-escalation and expansion study conducted at Guangdong Lung Cancer Institute, AC0010 demonstrated promising efficacy and good tolerability in advanced NSCLC patients with EGFR T790M-mediated resistance to previous EGFR TKIs, (NCT02330367). As disease progression (PD) with EGFR T790M-directed therapy also emerges over time, we investigated the resistance mechanisms to AC0010 in this study.
a9ded1e5ce5d75814730bb4caaf49419 Method
Serial ctDNA samples obtained from patients who developed PD with AC0010 were analyzed using ultra-deep sequencing capturing 295 cancer-related genes. Alterations that were absent before treatment and acquired at PD or that increased in abundance during treatment were identified as putative resistance mechanisms.
4c3880bb027f159e801041b1021e88e8 Result
Longitudinal plasma samples were obtained from 23 patients who progressed on AC0010 (data cut-off October 14, 2016; figure1). Putative resistance mechanisms to AC0010 were identified in 19/23 patients (>1 putative resistance mechanism was detected in some patients). EGFR amplification was the predominant resistance mechanism (21.1% [4/19 patients]), followed by TP53 loss of heterozygosity (15.8% [3/19]). EGFR C797S mutation, Met amplification and mutations in the PI3KCA pathway each occurred in 10.5% of patients (2/19). SCLC transformation, ERBB2 amplification, CD79A_A32G mutation, CDKN2A_R80 mutation, CRLF2 amplification, MLH1 amplification, Rb1 loss, and concurrent rise in the allelic fraction of tumor suppressor gene TP53 and Rb1 were each detected in 5.3% of patients (1/19). In a patient with PD following single-agent AC0010 and EGFR amplification as the putative resistance mechanism to AC0010, subsequent treatment with AC0010 plus nimotuzumab (EGFR monoclonal antibody) successfully overcame resistance, resulting in a response that lasted for 7.7 months.
8eea62084ca7e541d918e823422bd82e Conclusion
The resistance landscape to AC0010 appears to differ from that described previously with osimertinib. In this cohort of patients in China, EGFR amplification was the predominant resistance mechanism to AC0010 and could be potentially overcome by EGFR dual inhibition.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA24 - Genomic Evolution, KEAP 3 and More Non-Coding RNA (ID 928)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Biology
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 205 BD
-
+
MA24.01 - Genomic Evolution Trajectory Depicts Invasiveness Acquisition from Pre-invasive to Invasive Adenocarcinoma (ID 11840)
10:30 - 10:35 | Author(s): Qing Zhou
- Abstract
- Presentation
Background
Accumulation of molecular abnormalities may depict evolution trajectories of tumor initiation and development. However, the genomic profile of early stage adenocarcinoma and molecular mechanism of invasiveness acquisition from pre-invasive to invasive adenocarcinoma remains barely explored.
a9ded1e5ce5d75814730bb4caaf49419 Method
We simultaneously collected 20 patients with adenocarcinoma in situ (AIS) (n=5), minimally invasive adenocarcinoma (MIA) (n=5) and stage IA adenocarcinoma (lepidic/acinar predominant) (n=10). Whole exon sequencing (WES) was performed in pre-invasive adenocarcinoma with multi-region specimens and stage IA adenocarcinoma. Analysis of genomic alteration among different pathological status was performed and tumor mutation burden (TMB) was calculated as well as six mutation types individually. Enriched pathways of each pathology were measured through KEGG analysis.
4c3880bb027f159e801041b1021e88e8 Result
Baseline characteristics was generated through heatmap with smokers (2/20, 10%) and EGFR mutation (13/20, 65%) among whole population. AIS/MIA indicated much lower number of mutations than invasive adenocarcinoma (IAC) while TMB revealed the same trend without statistical significance. Multi-region sequencing showed high heterogeneity of single nucleotide variation (SNV) in AIS and MIA. Unique SNV presented dominant proportion in initial status. Cluster analysis showed higher copy number variation in AIS/MIA than IAC with cell adhesion molecules (CAMs) enriched in AIS/MIA while variety pathway enrichment in IAC through KEGG analysis. C>A transversions held major proportion in early stage adenocarcinoma and a significant increase in the proportion of C>T and C>G mutation was exhibited when evolving into IAC.
8eea62084ca7e541d918e823422bd82e Conclusion
Intratumor heterogeneity may occur in the very beginning of adenocarcinoma. High copy number variation was dominant event for AIS/MIA while higher tumor mutation burden was seen in IAC. Tobacco signature encompassing C>A transversions dominates the early development of adenocarcinoma and APOBEC signature may play a potential role in acquisition of cancer invasiveness.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 3
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-112 - Osimertinib vs Standard of Care (SoC) EGFR-TKI as First-Line Treatment in Chinese Patients With EGFRm Advanced NSCLC (ID 12211)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
Osimertinib is an irreversible, central nervous system (CNS) active EGFR-TKI, selective for both EGFRm and T790M resistance mutations. FLAURA (NCT02296125) is a PhIII, double-blind, randomized study assessing efficacy and safety of osimertinib vs SoC EGFR-TKI (erlotinib/gefitinib) in first-line patients with EGFRm advanced NSCLC. FLAURA results (556 patients, globally) are published. We present the China cohort results.
a9ded1e5ce5d75814730bb4caaf49419 Method
The cohort included self-identified Chinese patients, enrolled in China. Eligible patients: ≥18 years, Ex19del/L858R EGFRm advanced NSCLC, no prior EGFR-TKI/systemic anti-cancer therapy for advanced disease. Neurologically stable patients with CNS metastases were allowed, if definitive treatment/corticosteroids were completed ≥2 weeks before enrolment. Patients were randomized 1:1 to osimertinib 80 mg once daily (qd) orally or SoC EGFR-TKI (gefitinib 250 mg qd orally selected by all Chinese sites), stratified by mutation status (Ex19del/L858R). Primary endpoint: progression-free survival (PFS) by RECIST v1.1, per investigator. Data cutoff: 10/01/2018.
4c3880bb027f159e801041b1021e88e8 Result
Overall, 136 patients were randomized (osimertinib n=71; SoC n=65); 19 were also included in the global analysis. Baseline characteristics were balanced across arms (osimertinib/SoC): female 61/71%; smoking history 25/23%; WHO performance status 1 90/80%; Ex19del 51/51%, L858R 49/49%; CNS metastases 24/32%.
Efficacy endpoint Osimertinib
n=71SoC
n=65PFS events, total patients
(% maturity)40
(56%)51
(78%)PFS hazard ratio (HR)*
(95% CI)0.56 (0.37, 0.85); p=0.007 Median PFS, months
(95% CI)17.8
(13.6, 20.7)9.8
(8.3, 13.8)Objective response rate (ORR),
% (95% CI)83%
(72, 91)75%
(63, 85)Median duration of response (DoR), months
(95% CI)16.4
(12.3, NC)10.9
(8.3, 13.8)*A hazard ratio <1 favours osimertinib. PFS benefit was observed across all subgroups, irrespective of EGFR mutation status and including patients with/without CNS metastases at study entry. Median total treatment duration: osimertinib, 18.9 months; SoC, 13.6 months. No new safety signals were reported. Numerical increase in grade ≥3 AEs was reported in the osimertinib arm (49%) versus SoC arm (23%). Most grade ≥3 AEs in the osimertinib arm were investigator-reported laboratory and disease-related AEs; incidence of non-laboratory-related events was low. AEs leading to discontinuation: osimertinib, 13%; SoC, 6%. In the osimertinib arm, most AEs leading to discontinuation were fatal disease-related events.
8eea62084ca7e541d918e823422bd82e Conclusion
Osimertinib improved PFS vs SoC EGFR-TKI (HR: 0.56) as first-line treatment in Chinese patients with EGFRm advanced NSCLC, consistent with the global analysis.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-55 - Unique Genetic Profiles from Cerebrospinal Fluid Could Predict Survival of EGFR-Mutant NSCLC with Leptomeningeal Metastases (ID 12369)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
Leptomeningeal metastases (LM) are more frequent in NSCLC with EGFR mutations;and cerebrospinal fluid (CSF) could reveal the unique genetic profiles of LM in our previous studies, but whether they could predict the overall survival (OS) of LM remains unknown.
a9ded1e5ce5d75814730bb4caaf49419 Method
EGFR-mutant NSCLC patients with LM were enrolled,and clinical data and genetic profiles detected by Next-generation sequencing were collected. We further drew nomogram with endpoint of OS after LM, then performed index of concordance (C-index) and survival analysis to evaluate predictive role.
4c3880bb027f159e801041b1021e88e8 Result
In total, 61 patients were enrolled and all with genetic profiles from CSF. Patents with high copy number variations (CNVs) or harboring CDK6, TP53 exon5 or FGF19 in CSF demonstrated significant poorer OS than those without (Fig. 1). Cox regression analysis indicated CNVs, CDK6,CDKN2A,TP53,MET and NTRK1 as prognostic factors and further selected for nomogram (Fig. 2). C-index of nomogram was 0.743, indicating the moderate predictive effect. In the calibration curves, we scored the patients based on the model, using bisection and trisection methods to divide into low and high points groups; and low, medium and high points groups (Fig. 3), and significant difference were found in both the survival analyses (NA versus 7.47months, P<0.01) and (NA, 10.33 versus 4.43 months, P<0.01) respectively. Patients who received Osimertinib after LM seemed to have longer OS than those who did not (14.5 months versus 7.7 months) but without significant difference(P=0.10); however interestingly, in those with EGFR T790M negative who took Osimertinib after LM by themselves obtained survival benefit than those who did not(NA versus 7.7 months, P=0.04), and the results needed to be validated.
8eea62084ca7e541d918e823422bd82e Conclusion
Unique genetic profiles from CSF could well predict OS of LM. High CNVs, CDK6, TP53 exon5 or FGF19 in CSF in CSF may be related to poor prognosis of LM.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-97 - Cluster Trial: Ph2 Biomarker-Integrated Study of Single Agent Alpelisib, Capmatinib, Ceritinib and Binimetinib in advNSCLC (ID 12065)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
Several genetically altered signaling pathways have been profiled in NSCLC, enabling advanced management of NSCLC using targeted therapies. This study investigated the therapeutic spectrum of NSCLC with uncommon molecular alterations by allocating patients to treatment arms based on molecular aberrations; targeted therapies alpelisib (PI3Kαi), capmatinib (METi), ceritinib (ALKi), and binimetinib (MEKi) were evaluated.
a9ded1e5ce5d75814730bb4caaf49419 Method
The study was based on the umbrella design. Key objectives: investigate feasibility of using one trial for different agents based on biomarker-integrated analysis, assess anti-tumor activity, characterize safety, tolerability and PK profiles of individual agents. Key eligibility criteria: age ≥18 years; ECOG PS ≤2; failed prior treatment/unsuitable for chemotherapy. Documentation of locally determined molecular alterations before treatment allocation was required (alpelisib, 350 mg QD: PIK3CA mutation/amplification; capmatinib, 400 mg BID (tablet): MET IHC overexpression/amplification; ceritinib, 750 mg QD: ALK or ROS1 rearrangement; binimetinib, 45 mg BID: KRAS, NRAS or BRAF mutation).
4c3880bb027f159e801041b1021e88e8 Result
Sixty-six patients with advNSCLC were enrolled (median age 58 years; 65.2% male: alpelisib, n=2; capmatinib, n=16; ceritinib, n=26; binimetinib, n=22). As of Feb 28, 2018, 10 patients in ceritinib and 2 in binimetinib arms were ongoing. Twenty-four patients had confirmed partial responses (36.4%): alpelisib, 0%; capmatinib, 18.8%; ceritinib, 73.1%; binimetinib, 9.1% (Figure). Longest mPFS (14.4 months) was in ceritinib arm. Among the most common treatment-related AEs: alpelisib: malaise, hyperglycemia, dysgeusia; capmatinib: nausea, anemia, peripheral edema, decreased appetite; ceritinib: diarrhea, vomiting, ALT/AST elevation; binimetinib: mouth ulceration, AST, blood CPK increased, rash. Most AEs were grade 1/2.
8eea62084ca7e541d918e823422bd82e Conclusion
6f8b794f3246b0c1e1780bb4d4d5dc53
Objective responses/tumor shrinkage were observed in the study; highest ORR and mPFS were observed with ceritinib, although patient numbers differed between arms. All treatments were well tolerated; no new safety signals were observed. This study demonstrated the feasibility of an umbrella trial and importance of precision medicine in the management of advNSCLC with uncommon molecular alterations.
-
+
P1.03 - Biology (Not CME Accredited Session) (ID 935)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.03-34 - Combined Molecular and Radiological Evaluation Unveils Three Subtypes of Disease Progression to a Third Generation EGFR TKI (ID 12055)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
The definition of disease progression (PD) to EGFR TKIs has evolved from RECIST to a combination of clinical and RECIST evaluation. Patients with dramatic, local or gradual progression to third generation EGFR TKIs have been tailored to different subsequent treatment strategies. However, little is known about progression to third generation EGFR TKIs from molecular perspective.
a9ded1e5ce5d75814730bb4caaf49419 Method
Longitudinal plasma samples were collected from T790M-positive patients who progressed on a third generation EGFR TKI AC0010 in a phase I/II study in Guangdong Lung Cancer Institute. A pre-defined and unified molecular and radiological evaluation of PD were performed. Ultra-deep sequencing capturing 295 cancer-related genes was performed to track the changes in ctDNA to depict molecular PD, which was defined by acquired SNV/SCNV, or ≥20% increase in allelic fraction/copy number of pre-existing SNV /SCNV or both. Radiological PD was defined by RECIST.
4c3880bb027f159e801041b1021e88e8 Result
As of October 2016, 102 serial plasma samples from 23 patients with clinical PD were included. Three subtypes of PD to AC0010 were revealed (Fig1). Molecular PD occurred prior to radiological PD in 43.5% of patients (10/23), with an average lead time of 3.0 months. Molecular PD occurred concurrently with radiological PD in 39.1% of patients (9/23). Interestingly, 17.4% of patients (4/23) experienced radiological PD prior to molecular PD, with molecular PD occurred during AC0010 continuation beyond progression (CBPD) in 3 patients. Of patients experienced clinical stable PD in extracranial lesions, radiological PD occurring prior to molecular PD group (n=2) demonstrated longer duration of AC0010 CBPD than molecular PD occurring prior to (n=3) or concurrently with radiological PD groups (n=4) (Median, 5.6 months vs. 1.9 months vs. 1.8 months).
8eea62084ca7e541d918e823422bd82e Conclusion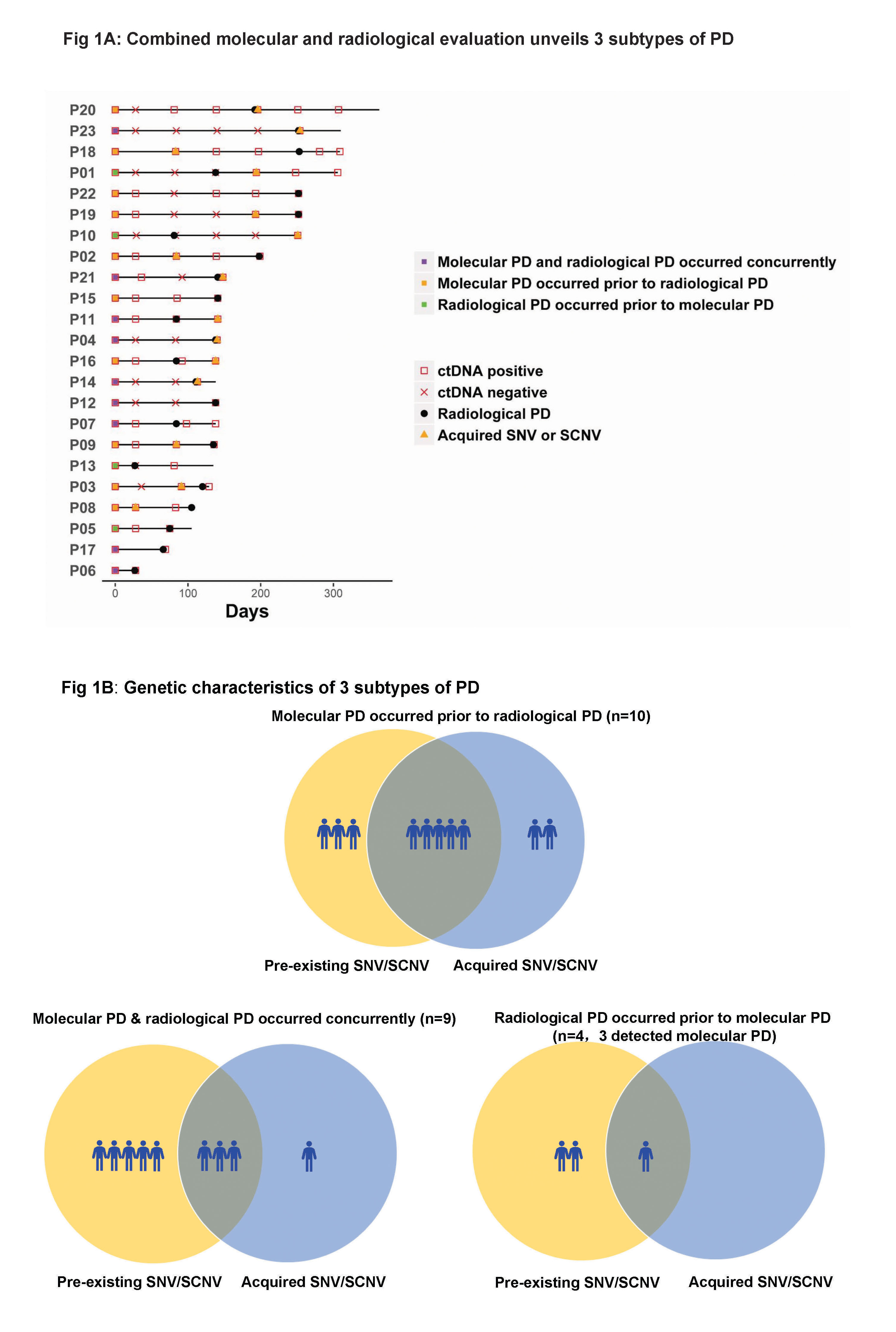
Our study revealed 3 distinct subtypes of PD to AC0010, providing insights into PD by combining molecular and radiological evaluation and might guide the optimal time for treatment switch and personalized subsequent treatments.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-52 - Identification of Leptomeningeal Metastasis-Specific Exosomal miRNA Signatures in Cerebrospinal Fluids of NSCLC Patients (ID 13074)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
Leptomeningeal metastasis (LM) is a devastating complication with poor prognosis in non-small-cell lung cancer (NSCLC) patients. The confirmed diagnosis of LM usually involves neurological evaluation, MRI imaging, and cytopathology analysis of limited tumor cells from cerebrospinal fluid (CSF). Exosomes are extracellular vesicles in body fluids enriched with microRNAs (miRNAs), which have been implicated to participate in brain metastasis. Here, we aimed to identify LM-specific exosomal miRNA signatures in NSCLC patients to elucidate their potential role in LM mechanism and to predict LM via liquid biopsy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Exosomes prepared from CSF and plasma samples of 39 advanced NSCLC patients with (LM+) or without (LM-) LM as well as 12 non-cancer individuals (NC) were underwent small RNA next-generation sequencing. For patients in the LM+ group, paired plasma samples were taken before (PLM+pre) and upon (PLM+post) LM diagnosis. Exosomal miRNA profiles were subjected for differential expression analysis, pathway enrichment analysis, and signature discovery.
4c3880bb027f159e801041b1021e88e8 Result
Unsupervised hierarchical clustering of the miRNA expression profiles clearly separated CSF samples into LM+ and LM free groups (LM- and NC). Interestingly, these samples were stratified based on their LM status only, regardless of their intraparenchymal metastasis status. In total, 247 (185 up and 62 down-regulated) miRNAs were identified differentially presented in the LM+ CSF exosome samples compared to the LM- and NC groups. Top altered miRNAs include dramatically up-regulated miR-200 family and down-regulated miR-144/451 cluster. Predicted gene targets of these top-regulated miRNAs were significantly enriched in Ras/MAPK/PI3K-AKT signaling, endocytosis pathways, and so on. Promisingly, a signature of five CSF exosomal miRNAs (let-7e-5p, miR-28-3p, miR-375, miR-200a-3p, and miR-486-5p) was identified for classification of LM+ patients with 100% sensitivity and 100% specificity. Due to the higher background complexity, we only identified one miRNA (miR-24-3p) was significantly up-regulated and one miRNA (miR-92b-5p) was significantly down-regulated in LM+ patients’ plasma-derived exosomes (PLM+pre and PLM+post) compared with the LM free group (PLM- and PNC). However, a combined signature of seven miRNAs (miR-24-3p, miR-223-3p, miR-340-5p, miR-27a-3p, miR-423-5p, miR-2110 and miR-342-5p) from PLM+pre samples was identified for the prediction of future LM with 81% sensitivity and 76% specificity.
8eea62084ca7e541d918e823422bd82e Conclusion
NSCLC patients with LM present a remarkably distinct CSF exosomal miRNA signature, which may involve in the progression of LM, and can be used as diagnostic biomarkers for LM. Furthermore, the identification miRNA signature in the pre-LM plasma samples suggests the potential use of liquid biopsy to predict LM for better patient care.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.04 - Immunooncology (Not CME Accredited Session) (ID 953)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.04-29 - Preliminary Results With Tislelizumab in Chinese Patients With Non-Small Cell Lung Cancer (NSCLC) (ID 11319)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
NSCLC accounts for 80–85% of all lung cancers and has a poor prognosis at later stages. Immune checkpoint inhibitors have shown efficacy in patients (pts) with advanced NSCLC. Tislelizumab is a humanized IgG4 monoclonal antibody with high affinity/specificity for PD-1. Tislelizumab was specifically engineered to minimize FcϒR binding on macrophages that, based on preclinical evidence, is believed to minimize potentially negative interactions with other immune cells. In a phase 1 study, tislelizumab was generally well tolerated and showed antitumor activity in NSCLC pts; 200 mg IV Q3W was established as the recommended tislelizumab dose.
a9ded1e5ce5d75814730bb4caaf49419 Method
In the ongoing indication-expansion phase of this study, Chinese pts with histologically confirmed NSCLC were enrolled into PD-L1-high (PD-L1+; ≥10% tumor cells expressing PD-L1) and PD‑L1‑low (PD-L1–) cohorts. Antitumor activity (RECIST v1.1) and safety/tolerability (NCI-CTCAE v4.03) were assessed.
4c3880bb027f159e801041b1021e88e8 Result
As of 8 Dec 2017, 42 NSCLC pts (median age 54 yr [range 37–72]) were enrolled; 17 were PD-L1+ and 25 were PD-L1–. Most pts were male (69%), former/current smokers (57%), and had received prior therapy (95%). Adenocarcinoma was the most prevalent histology (57%). Median follow-up was 4.5 mo and 23 pts remain on treatment. Of the 39 response-evaluable pts, 4 (n=2/14, PD-L1+; n=2/25, PD-L1–) achieved confirmed PR and 20 (n=6/14, PD-L1+; n=14/25, PD‑L1–) achieved SD, including 4 (n=2, PD-L1+; n=2, PD‑L1–) with unconfirmed PR. Across the study population, ORR was 10% and DCR was 61.5%. ORRs by cohort were 14% (PD‑L1+) and 8% (PD-L1–), respectively. Common treatment-related AEs were increased AST (24%), increased ALT (19%), hypothyroidism (12%), and rash (12%). Five grade ≥3 treatment-related AEs occurred in 4 pts (increased AST [n=2], hyperglycemia, increased ALT, and increased GGT [n=1 each]). No treatment-related grade 5 events were reported.
8eea62084ca7e541d918e823422bd82e Conclusion
Tislelizumab was generally well tolerated and demonstrated antitumor activity in previously treated pts with advanced NSCLC. A global phase 3 study (NCT03358875) of tislelizumab vs docetaxel as potential second/third-line therapy in NSCLC pts who progressed after a platinum-based regimen is ongoing.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.04-30 - PD-1/PD-L1 Inhibition Might be an Option for the Treatment of Advanced Primary Pulmonary Lymphoepithelioma-Like Carcinoma (ID 12698)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a rare subtype of non-small-cell lung cancer mostly reported in Asian countries, which is frequently associated with Epstein-Barr virus (EBV) infection. There is no consensus on the choice for the treatment of advanced primary pulmonary LELC. The utility of PD-1/PD-L1 inhibitor to this cancer type remains poorly understood.
a9ded1e5ce5d75814730bb4caaf49419 Method
From January 2008 to April 2017, a total of 53 patients receiving surgical removal and diagnosed as primary pulmonary LELC in Guangdong Lung Cancer Institute (GLCI) were enrolled in this study. Sections formalin-fixed and paraffin-embedded (FFPE) tumor samples were stained with PD-L1 antibody (clone E1L3N, Cell Signaling Technology) by immunohistochemistry (IHC). PD-L1 expression more than 1% in tumor cells was considered as PD-L1 positive. Moreover, we reviewed the medical records of 13 primary pulmonary LELC patients who received the treatment of PD-1/PD-L1 inhibitors in GLCI. Their clinicopathological characteristics and relevant prognostic data were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
Among the 53 patients with operable disease, the median age was 56 (36-78), there were 26 males and 27 females, 15 smokers and 38 nonsmokers. Positive rates of PD-L1 in the early pulmonary LELC were 78.8% (41/52,one specimen can't evaluate) and 73.1% (38/52) and 23.1% (12/52) respectively at the cutoff values of 1% and 5% and 50% positivity in tumor cells. ORR of PD-1/PD-L1 inhibition in the evaluable 12 patients with advanced LELC was 16.7% (2/12), and DCR was 75.0% (9/12). In the 6 patients with positive PD-L1 expression, ORR was 33.3% (2/6), DCR was 100.0% (6/6). The two responder patients got 55% and 64% shrinkage of the tumors respectively. All patients had no EGFR mutations.

8eea62084ca7e541d918e823422bd82e Conclusion
This preliminary study showed that pulmonary LELCs have remarkably high incidence of PD-L1 expression. PD-1/PD-L1 inhibitors might be an option for the treatment of advanced primary pulmonary LELC, which needs further investigation.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 961)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.12-11 - A Prognostic Model Integrating Immunohistochemistry Markers for Extensive-Disease Small-Cell Lung Cancer (ID 11828)
16:45 - 18:00 | Author(s): Qing Zhou
- Abstract
Background
Extensive-disease small-cell lung cancer (ED-SCLC) is a subtype of high-grade neuroendocrine carcinoma (HGNEC) with poor prognosis. We tend to build a prognostic nomogram and illustrate the failure pattern of first line etoposide/Irinotecan with paclitaxel (EP/IP) treatment.
a9ded1e5ce5d75814730bb4caaf49419 Method
250 ED-SCLC patients received first line EP/IP treatment were enrolled. Cox regression analysis was used to identify the prognostic factors to establish nomogram. The predictive accuracy of nomogram was evaluated by concordance index (C-index). Further stratification based on Ki67 and brain metastasis was performed through X-tile plot and Kaplan Meier.
4c3880bb027f159e801041b1021e88e8 Result
Cox regression analysis indicated brain metastasis as the prognostic factor and we further selected NSE, gender, TTF-1, Syn, tumor size and smoking status under clinical consideration for nomogram. C-index of nomogram suggested 0.65 with moderate predictive effect. Subgroup analysis showed patients with Ki67 lower than 85% had poorer prognosis than those over 90% (HR 0.59, 95%CI 0.39-0.92, p=0.02). Those without brain metastasis at baseline achieving partial response (PR)/complete response (CR) suggested no prognostic significance in brain progression compared to other progression group.
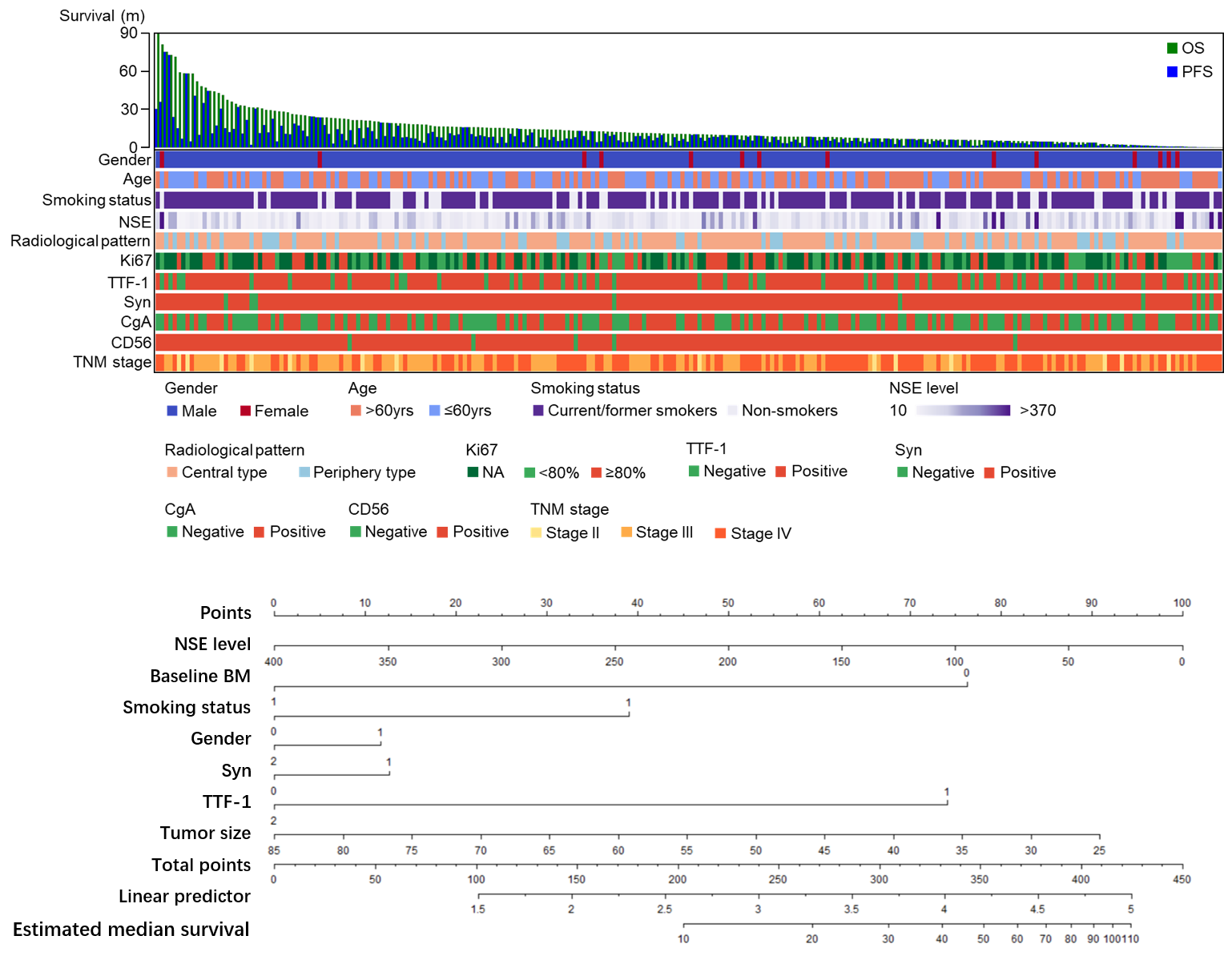
8eea62084ca7e541d918e823422bd82e Conclusion
Established nomogram could well predict prognosis in ED-SCLC. Ki67 might play a potential role in prognosis of SCLC. Application of preventive cranial irradiation might be challenged in ED-SCLC patients without brain metastasis.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-113 - A Multicenter Survey of One Year Survival Among Chinese Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer (CTONG1506) (ID 12337)
12:00 - 13:30 | Presenting Author(s): Qing Zhou
- Abstract
Background
Previous results of CTONG1506 study showed that gene aberration test rate was increasing in Chinese NSCLC patients and first-line treatment was standardized accordingly. This survey further described one year survival of patients with different gene aberration status and under different first-line treatments.
a9ded1e5ce5d75814730bb4caaf49419 Method
CTONG1506 was a two-year series cross-sectional study. Patients with advanced nonsquamous NSCLC who were admitted from August 2015 to March 2016 and who received first-line anti-cancer treatment at one of 12 tertiary hospitals across China were included. Data extracted from medical charts were entered into medical record abstraction forms, which were collated for analysis. Survival information was collected one year after patients were admitted to hospital. One year survival rate and its 95% confidence interval were analysed by Kaplan-Meier method.
4c3880bb027f159e801041b1021e88e8 Result
A total of 707 patients were analysed, with mean age of 57 years and 56.7% were male. Among the 487 patients who had survival data, 192 were EGFR- mutation positive (86 mutated in exon 19 [one year survival rate 0.90, 95% CI: 0.81-0.94] and 88 mutated in exon 21 [one year survival rate 0.84, 95% CI: 0.75-0.90]), 27 patients were ALK positive and 164 patients were EGFR and ALK wild type. Most EGFR mutation positive patients (128/192) received tyrosine kinase inhibitors (TKIs) as first-line treatment and most EGFR wild type patients (155/175) received first-line chemotherapy (Chemo). Pemetrexed was the most common non-platinum chemotherapy-backbone agent (120/155) in platinum doublet regimens. One year survival rates are shown in the table.
8eea62084ca7e541d918e823422bd82e Conclusion
This national-wide real world study of tertiary hospitals in China revealed that a majority of (>75%) advanced nonsquamous NSCLC patients survived more than one year and was comparable to well-controlled clinical trial results, indicating survival benefits by gene aberration status guided standard of care. This result may be further validated by our on-going two-year survey.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
PC09 - Approaches to Management of Advanced NSCLC (ID 848)
- Event: WCLC 2018
- Type: Pro-Con Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 107
-
+
PC09.01 - Debate 1: Early vs Delayed Treatment of Asymptomatic Brain Metastases in Wild-Type NSCLC - Early (ID 11639)
10:30 - 10:45 | Presenting Author(s): Qing Zhou
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.


