Virtual Library
Start Your Search
Ben J Solomon
Author of
-
+
MA04 - Novel Approaches with IO (ID 900)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Immunooncology
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 13:30 - 15:00, Room 107
-
+
MA04.03 - Immunotherapy for Non-Small Cell Lung Cancers (NSCLC) with Oncogenic Driver Mutations: New Results from the Global IMMUNOTARGET Registry (ID 13187)
13:40 - 13:45 | Author(s): Ben J Solomon
- Abstract
- Presentation
Background
Prospective data on immunotherapy for NSCLC with oncogenic driver mutations are limited. We recently reported first results from the global IMMUNOTARGET registry (Mazières, ASCO 2018). Here, we present new data for PD-L1 and mutation subgroups.
a9ded1e5ce5d75814730bb4caaf49419 Method
In 2017, we started an international retrospective registry study ("IMMUNOTARGET") for patients with advanced NSCLC, known driver mutations (KRAS, EGFR, ALK, ROS1, BRAF, HER2, MET and RET) and PD-L1 immune checkpoint inhibitor therapy. The registry is approved by University of Toulouse and Swissethics, and funded by University of Toulouse and Cantonal Hospital of Lucerne. Anonymized real-world data submitted to the coordinating center include: patient and tumor characteristics, mutation test methods and results, systemic therapy lines, immune related adverse events, best response by RECIST, survival, and tumor PD-L1 expression (optional). Statistical calculations including best response, median PFS and OS are done at University of Toulouse.
4c3880bb027f159e801041b1021e88e8 Result
In April 2018, the registry included 551 pts from Europe, USA, Israel and Australia. Patients were 50% male/female, 28% current smokers, median age 60 years (range 28-83), 85% had PS0/1. Most (73%) tumors were stage IV at diagnosis, almost all (96%) were adenocarcinomas. Molecular classification by dominant driver mutation: KRAS=271 (49%), EGFR=125 (23%), BRAF=43 (8%), MET=36 (7%), HER2=29 (5%), ALK=23 (4%), RET=16 (3%), ROS1=7 (1%), 1 (0.2%) not classified (ALK+RET+MET). Most pts received nivolumab (466) or pembrolizumab (48) and were treated with immunotherapy in second or third line (67%). The median number of cycles was 5 (range 1-68). Fifty (11%) pts had grade 3-5 toxicity. Median OS from start of immunotherapy was 13.3 months, median PFS was 2.8 months. Best response was PR/CR in: KRAS=26%, BRAF=24%, ROS1=17%, MET=16%, EGFR=12%, HER2=7%, RET=6%, ALK=0%. Percentage of PD-L1 positive cells was available for 177 pts: 0%=71 (40%), 1-49%=46 (26%), 50-100%=60 (34%). Median % of positive cells was highest for ROS1 (90%), BRAF (50%), MET (30%) and RET (26%) mutant tumors. PD-L1 positivity was predictive for improved PFS in KRAS and EGFR mutant tumors. PD-L1 status was known in 18 tumors with ALK, ROS1 or RET rearrangements: 5 had 0%, 4 had 1-49% and 9 had 50%-100%. No tumor remissions were observed in this subgroup. The registry remains open, updated results will be presented at the conference.
8eea62084ca7e541d918e823422bd82e Conclusion
Although response rates were lower than in KRAS mutant NSCLC, individual tumors with other driver mutations responded to immunotherapy. PD-L1 expression may not accurately predict clinical benefit from immunotherapy in some molecular subgroups, better markers are needed.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA16 - Novel Mechanisms for Molecular Profiling (ID 917)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 203 BD
-
+
MA16.07 - Discussant - MA 16.04, MA 16.05, MA 16.06 (ID 14646)
14:10 - 14:25 | Presenting Author(s): Ben J Solomon
- Abstract
- Presentation
Abstract not provided
Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA02 - Novel Therapies in ROS1, HER2 and EGFR (ID 893)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 105
-
+
OA02.03 - Clinical Activity of Lorlatinib in Patients with ROS1+ Advanced Non-Small Cell Lung Cancer: Phase 2 Study Cohort EXP-6 (ID 12787)
10:50 - 11:00 | Author(s): Ben J Solomon
- Abstract
- Presentation
Background
Among patients with ROS1-positive non-small cell lung cancer (NSCLC), most achieve initial benefit from crizotinib treatment but often develop resistance, and further treatment options are limited. Lorlatinib is a potent, brain-penetrant third-generation ALK/ROS1 TKI with broad mutational coverage. It has shown compelling clinical activity in patients with ALK-positive and ROS1-positive advanced NSCLC, most of whom had CNS metastases and had received prior crizotinib.
a9ded1e5ce5d75814730bb4caaf49419 Method
This ongoing Phase 2 study (NCT01970865) enrolled patients with ROS1-positive advanced NSCLC ± asymptomatic CNS metastases without restriction on the type or number of prior lines of therapy (cohort EXP-6). Patients received lorlatinib 100 mg QD. Primary endpoints were overall and intracranial response by independent central review. Secondary endpoints included duration of response and progression-free survival. Safety was assessed in all treated patients (cohorts EXP-1–6); molecular profiling is ongoing.
4c3880bb027f159e801041b1021e88e8 Result
As of the data cut-off (02 Feb 2018), 47 patients with ROS1+ NSCLC were treated; 25 had baseline CNS metastases; 34 had received prior crizotinib and 13 were crizotinib-naïve. Treatment with lorlatinib led to rapid and durable responses in both crizotinib-naïve and crizotinib-pre-exposed patients (Table).
ICR-assessed endpoint Crizotinib-naïve Crizotinib-pre-exposed Total EXP-6 Overall, N 13 34 47 ORR, % (95% CI) 61.5 (31.6, 86.1) 26.5 (12.9, 44.4) 36.2 (22.7, 51.5) Confirmed response, n 8 9 17 Response lasting at least 12 months, n
5 5 10 Median time to tumor response, months (range) 1.4 (1.3–8.3) 2.5 (1.4–4.2) 1.4 (1.3–8.3) Intracranial (IC), N 6 19 25 IC ORR, % (95% CI) 66.7 (22.3, 95.7) 52.6 (28.9, 75.6) 56.0 (34.9, 75.6) Confirmed IC response, n 4 10 14 IC response lasting at least 12 months, n
1 4 5 Median PFS, months (95% CI)a 21.0 (4.2, 26.7) 8.5 (4.4, 18.0) 9.9 (5.5, 21.0) ICR, independent central review; PFS, progression-free survival.
aPer Kaplan-Meier method.The most common treatment-related adverse events (TRAEs) in EXP-6, were hypercholesterolemia (83%) and hypertriglyceridemia (60%). In EXP-6, 36% and 23% of patients had TRAEs leading to dose interruptions and dose reductions, respectively. No permanent treatment discontinuations due to TRAEs or treatment-related deaths occurred.
8eea62084ca7e541d918e823422bd82e Conclusion
Lorlatinib showed clinically meaningful benefit in patients with ROS1-positive NSCLC, including those who had received prior crizotinib or were crizotinib-naive, as demonstrated by rapid and durable responses. These findings further suggest that the activity of lorlatinib differs depending on prior exposure to crizotinib. The safety profile of lorlatinib in ROS1 patients was comparable to that previously reported in the overall ALK/ROS1 population.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA12 - Novel Therapies in MET, RET and BRAF (ID 921)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 15:15 - 16:45, Room 106
-
+
OA12.02 - Updated Antitumor Activity of Crizotinib in Patients with MET Exon 14-Altered Advanced Non-Small Cell Lung Cancer (ID 13453)
15:25 - 15:35 | Author(s): Ben J Solomon
- Abstract
- Presentation
Background
MET exon 14 alterations occur in ~3% of non-squamous non-small cell lung cancer (NSCLCs) and 20–30% of sarcomatoid lung carcinomas. Here we present updated antitumor activity for crizotinib in patients with advanced NSCLC whose tumors are positive for MET exon 14 alterations (hereafter MET exon 14-positive NSCLC), including updated biomarker analyses in circulating tumor DNA (ctDNA).
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with MET exon 14-positive NSCLC by local molecular profiling were treated with 250 mg crizotinib BID in an expansion cohort of the ongoing PROFILE 1001 study (NCT00585195). Responses were based on derived investigator assessment per RECIST v1.0. Prospective plasma profiling for MET exon 14 alterations in plasma ctDNA was performed (PlasmaSELECT-R64; Personal Genome Diagnostics, Boston, MA).
4c3880bb027f159e801041b1021e88e8 Result
As of Jan 31, 2018, 69 patients (65 response-evaluable) with MET exon 14-positive NSCLC had been treated. Median age was 72 y (range: 34, 91). Tumor histology was: 84% adenocarcinoma, 9% sarcomatoid adenocarcinoma, 4% squamous cell carcinoma and 3% adenosquamous carcinoma. 61% were former-smokers, 38% never-smokers and 1% a current smoker. Median duration of treatment was 7.4 mo (95% CI: 5.5, 9.1), with 29% of patients ongoing. Confirmed responses were 3 CRs and 18 PRs (ORR, 32% [95% CI: 21, 45]); 29 patients had SD as their best overall response (Figure).
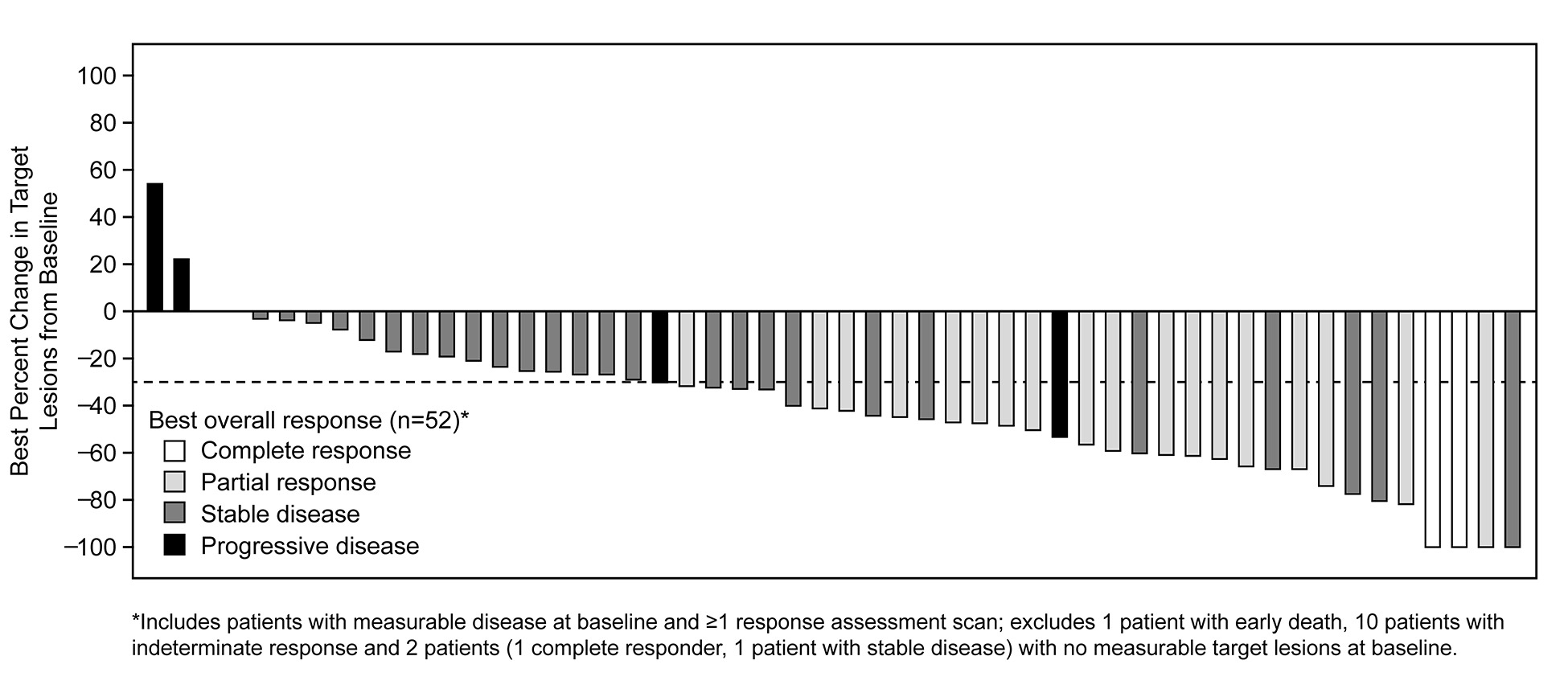
Median time to response was 7.6 weeks (range: 3.7, 16.3). Median DOR was 9.1 mo (95% CI: 6.4, 12.7). Median PFS was 7.3 mo (95% CI: 5.4, 9.1). MET exon 14 alterations were detected in ctDNA from 18/37 (49%) patients with analyzable samples.
8eea62084ca7e541d918e823422bd82e Conclusion
In patients with MET exon 14-positive advanced NSCLC, crizotinib treatment led to objective responses that were rapid and durable, with CRs in some cases. Plasma ctDNA profiling detected MET exon 14 alterations in a subset of patients who harbor MET exon 14 alterations by tumor testing.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA12.07 - Clinical Activity of LOXO-292, a Highly Selective RET Inhibitor, in Patients with RET Fusion+ Non-Small Cell Lung Cancer (ID 13922)
16:20 - 16:30 | Author(s): Ben J Solomon
- Abstract
- Presentation
Background
RET kinase gene fusions are actionable drivers that occur in ~2% of non-small cell lung cancers (NSCLC). However, the clinical activity of multikinase inhibitors (MKIs) with anti-RET activity in RET fusion+ NSCLC patients has been limited. LOXO-292 is a highly selective RET inhibitor, with preclinical activity against diverse RET fusions, potential acquired resistance mutations, and against brain metastases.
a9ded1e5ce5d75814730bb4caaf49419 Method
LIBRETTO-001 is a multicenter global phase 1/2 study (26 sites, 9 countries) enrolling patients w/ advanced solid tumors (NCT03157128) including RET fusion+ NSCLC. Patients are dosed orally in 28-day cycles with dose escalation following a 3+3 design. The primary endpoint is MTD/recommended dose determination. Secondary endpoints include safety, overall response rate (ORR, RECIST 1.1) and duration of response (DoR). Initial data were presented at the ASCO 2018 Annual Meeting.
4c3880bb027f159e801041b1021e88e8 Result
As of 02-April 18, 82 solid tumor patients (including 38 RET fusion+ NSCLC) were treated at 8 doses (20 mg QD-240 mg BID). The MTD was not reached. AEs (≥10% of patients) were fatigue (20%), diarrhea (16%), constipation (15%), dry mouth (12%), nausea (12%), and dyspnea (11%); most were grade 1-2. 2 TEAEs ≥ grade 3 were attributed to LOXO-292 (Gr3 tumor lysis syndrome, Gr3 increased ALT). Of the 38 RET fusion+ NSCLC pts, 30 had at least 1 post-baseline assessment or discontinued LOXO-292 prior to such assessment. 26 of 30 patients (87%) had >20% radiographic tumor reduction (range: -21 to -72%). The ORR was 77% (23/30, 3 responses pending confirmation) with a confirmed ORR of 74% (20/27, excluding 3 patients with unconfirmed responses). The response rate was similar regardless of prior MKI treatment (12/15 MKI-naïve, 11/15 MKI pretreated). Responses occurred independent of upstream fusion partner when known (13/16 KIF5B vs 9/11 other) and included patients w/ baseline brain metastases. Most patients remained on treatment (33/38), including all responders. The median DoR was not reached (longest response was the first responder: >10+ months). Rapid plasma clearance of RET variants was observed, with complete clearance by day 15 in 10 of 17 (59%) NSCLC patients with assessable baseline and day 15 ctDNA.
8eea62084ca7e541d918e823422bd82e Conclusion
LOXO-292 was well-tolerated and had marked antitumor activity in RET-fusion+ NSCLC patients, including those w/ resistance to prior MKIs and brain metastases. Phase 2 cohorts are now open globally (160 mg BID). Updated safety and efficacy data as of 19 Jul 2018 will be presented.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-06 - First-Line Lorlatinib Versus Crizotinib for Advanced Anaplastic Lymphoma Kinase-Positive (ALK<sup>+</sup>) Non-Small Cell Lung Cancer (ID 12773)
16:45 - 18:00 | Author(s): Ben J Solomon
- Abstract
Background
Lorlatinib and crizotinib are oral tyrosine kinase inhibitors with activity against ALK and ROS1 fusion proteins. Crizotinib is well tolerated and has superior efficacy compared to chemotherapy for treatment of patients with advanced ALK+ non-small-cell lung cancer (NSCLC). However, resistance to crizotinib can develop, and the central nervous system (CNS) is often a site of disease relapse. Second-generation ALK inhibitors, ceritinib and alectinib, have demonstrated activity in crizotinib-naive or resistant treatment settings, and alectinib has been shown to have superior progression-free survival (PFS) compared to crizotinib as first-line therapy. Lorlatinib is a selective, CNS-penetrant ALK inhibitor that has potent activity against ALK and kinase domain resistance mutations, including the difficult-to-treat G1202R mutation.Lorlatinib has shown clinical activity in patients previously treated with crizotinib and other ALK inhibitors, including patients with progressive CNS metastases. This study aims to determine if lorlatinib is superior to crizotinib in prolonging PFS in treatment-naïve patients and to identify candidate biomarkers predictive of clinical efficacy or treatment resistance.
a9ded1e5ce5d75814730bb4caaf49419 Method
Trial Design
This global, multicenter, open-label phase 3 study will enroll ~280 treatment-naïve patients. Eligible patients must be aged ≥18 years, have Eastern Cooperative Oncology Group performance status of 0–2 and ≥1 measurable extracranial target lesion not previously treated with radiotherapy. Patients with asymptomatic brain metastases are eligible. Patients will be randomized (1:1) to lorlatinib 100 mg once daily or crizotinib 250 mg twice daily and stratified by presence of brain metastases (yes/no) and ethnicity (Asian/non-Asian). Treatment will continue until disease progression, patient refusal, or unacceptable toxicity. Crossover between treatment arms will not be permitted. The primary endpoint is PFS based on blinded independent central review (BICR) using RECIST v1.1. Secondary endpoints include PFS based on investigator assessment, overall survival, objective response (OR) by BICR and investigator assessment; intracranial (IC) OR (periodic magnetic resonance imaging will be performed for central nervous system evaluation), IC time to progression, duration of response and time to response all by BICR; tumor tissue and peripheral blood circulating free DNA biomarker assessment, adverse events and patient-reported health-related outcomes as assessed by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core-30 EORTC (QLQ-C30) and EORTC Lung Cancer Module (QLQ-LC13), and the 5-level EuroQol 5-dimension questionnaire (EQ-5D-5L). The first patient was screened on April 14, 2017. This study is registered with ClinicalTrials.gov as NCT03052608.
4c3880bb027f159e801041b1021e88e8 Result
Section not applicable
8eea62084ca7e541d918e823422bd82e Conclusion
Section not applicable
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.17 - Treatment of Locoregional Disease - NSCLC (Not CME Accredited Session) (ID 966)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.17-27 - IMpower030: Phase III Study Evaluating Neoadjuvant Treatment of Resectable Stage II-IIIB NSCLC with Atezolizumab + Chemotherapy (ID 12087)
16:45 - 18:00 | Author(s): Ben J Solomon
- Abstract
Background
A standard of care for resectable early-stage non-small cell lung cancer (NSCLC) is surgery alone or in combination with adjuvant or neoadjuvant platinum-based doublet chemotherapy (PT-DC). Nevertheless, 30%-70% of patients develop recurrence and die due to disease progression, highlighting the unmet need for more efficacious treatment regimens. Atezolizumab, an anti–programmed death-ligand 1 (anti–PD-L1) antibody that reinvigorates the anti-cancer immune response, has shown efficacy as monotherapy and in combination with chemotherapy in advanced NSCLC. On the basis of this activity, it is thought that the combination of atezolizumab and PT-DC may provide clinical benefit in the neoadjuvant setting by enhancing cancer cell killing and eradicating micro-metastases prior to surgery, thereby reducing the risk of disease recurrence. The objective of IMpower030 (NCT03456063) is to evaluate the efficacy and safety of atezolizumab in combination with PT-DC as neoadjuvant treatment for patients with resectable early-stage NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
IMpower030 is a global, Phase III, multicenter, double-blind, randomized study in patients with histologically or cytologically confirmed, resectable stage II, IIIA, or select IIIB (T3N2) NSCLC (per AJCC/UICC, 8th ed). Study inclusion requires measurable disease per RECIST v1.1, ECOG PS of 0/1, and eligibility for R0 resection with curative intent and PT-DC. Patients who have received prior therapy for lung cancer or who present with non-squamous NSCLC with activating EGFR mutations or ALK translocation are excluded. Approximately 302 patients will be randomized to receive 4 cycles of neoadjuvant atezolizumab (1200 mg Q3W, Arm A) or placebo (Arm B) in combination with an investigator-selected PT-DC regimen. Following surgical resection and pathology response assessment, treatment assignment will be unblinded; patients in Arm A will receive adjuvant treatment with atezolizumab for up to 16 cycles or until disease recurrence or unacceptable toxicity, whereas patients in Arm B will receive best supportive care and scheduled observational follow-up. The primary efficacy endpoint is major pathological response, defined as ≤ 10% residual viable tumor tissue at time of resection as assessed by an independent central pathology laboratory. Secondary efficacy endpoints include OS, ORR, investigator-assessed event-free survival and disease-free survival per RECIST v1.1, pathological complete response and patient-reported outcomes. Exploratory biomarkers will also be evaluated.
4c3880bb027f159e801041b1021e88e8 Result
Section not applicable
8eea62084ca7e541d918e823422bd82e Conclusion
Section not applicable
6f8b794f3246b0c1e1780bb4d4d5dc53


