Virtual Library
Start Your Search
Gilberto de Lima Lopes
Author of
-
+
MA19 - Genomic Markers of IO Response (ID 922)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Immunooncology
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 15:15 - 16:45, Room 201 BD
-
+
MA19.04 - The Clinical Implication of Frameshift Indel Mutation Burden in Non-Small Cell Lung Cancer (NSCLC) (ID 12592)
15:35 - 15:40 | Author(s): Gilberto de Lima Lopes
- Abstract
- Presentation
Background
Tumor mutational burden (TMB) has been proposed as a potential predictive marker for immune checkpoint inhibitor (ICI) response in many cancers, including NSCLC. Recently, research has revealed frameshift indel (fsindel) of all mutations to be significantly associated with ICI response in melanoma patients. However, little is currently known regarding its clinical implication in NSCLC patients treated with PD1/PD-L1 inhibitors (ICIs).
a9ded1e5ce5d75814730bb4caaf49419 Method
Next generation sequencing of 324 genes (FoundationOneTM) was used to derive fsindel burden and TMB. A total of 128 patient data with NSCLC treated with ICIs were analyzed from Northwestern University (N=68) and the University of Miami (N=60). A total of 128 patients were divided into two groups with 0 fsindel (FS-) and more than 1 fsindel (FS+). Progression free survival (PFS) and overall survival (OS) were compared between FS+ and FS- groups. PFS and OS outcomes of TMB high group (H-TMB, upper¼) and TMB low group (L-TMB, lower¼) were also compared.
4c3880bb027f159e801041b1021e88e8 Result
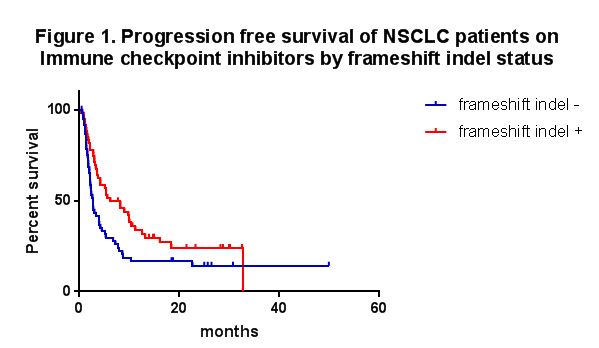
Among 128 patients, 51.6% belonged to FS+ group (N=66). Between FS-/FS+ groups, there were no significant differences in mean age (66.2/66.0) and performance status (0.9/0.9). Lines of ICIs used in the FS-/FS+ groups were 1st (19/19%), 2nd (47/56%), 3rd (24/11%), and 4th line or more (10/14%). FS+ group had significantly longer PFS compared with FS- group (median 6.2/2.7 months, P=0.02, Figure 1). No significant difference in OS was seen between the two groups (median 16.8/11.2 months, P=0.70). In contrast, however, H-TMB did not show any significant difference in PFS (median 5.6/4.0 months, P=0.14) and OS (median 15.8/15.1 months, P=0.69) compared to L-TMB.
8eea62084ca7e541d918e823422bd82e Conclusion
This is the first report to illustrate an association between fsindel and outcome in patients with NSLC treated with ICIs. Our findings suggest its potential role as a predictive marker for immunotherapy.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA04 - Improving Access and Outcomes in Lung Cancer Management (ID 898)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Nursing and Allied Professionals
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 201 F
-
+
OA04.01 - What is the Cost of a Strong Evidence for the Treatment of Advanced Non-Small Cell Lung Cancer? (ID 14410)
10:30 - 10:40 | Author(s): Gilberto de Lima Lopes
- Abstract
- Presentation
Background
Evidence-based medicine was developed to guide medical decisions based upon the strongest scientific evidence available in the literature. However, large randomized clinical trials are expensive. In addition, new antineoplastic drugs development is also extremely expensive. Therefore, we hypothesized that the strongest evidence available nowadays comes from studies developed by the pharmaceutical industry.
a9ded1e5ce5d75814730bb4caaf49419 Method
We carried out a search on network databases for studies published between 2014 and 2017. We included only experimental studies that assessed the treatment for advanced or metastatic non-small cell lung cancer. All included studies were divided into two groups: studies funded by pharmaceutical industry and studies funded by other sources. The primary end point was to compare the evidence strength of each group. Secondary end points were to compare other aspects, such as the number of patients included by each group of studies and the number of innovative drugs studied by each group of studies.
4c3880bb027f159e801041b1021e88e8 Result
We found 1,502 studies and included 299 studies (154 sponsored by pharmaceutical industry and 145 funded by other sources). 52,988 patients were included in all studies (36,455 in studies sponsored by industry and 16,533 in studies with other funding sources; p < 0.001). The studies funded by pharmaceutical industry had the stronger evidence compared with studies with other sources of funding (p = 0.005). Moreover, studies sponsored by pharmaceutical industry studied more innovative therapies (72.4% versus 48.9%; p < 0.001) and had a higher proportion of open access manuscript (60.8% versus 43.9%; p = 0.004). Results are summarized in the table.
Parameter Industry Sponsored P value Yes
154 (100%)
No
145 (100%)Number of patients 36,455 16,533 <0.001 Mean N of patients 236.7 115.6 Line First 110 (71.4%) 94 (64.8%) 0.220 Second or more 44 (28.6%) 51 (35.2%) Biomarker Yes 55 (35.9%) 55 (37.9%) 0.723 No 98 (64.1%) 90 (62.1%) Innovative Tx Yes 110 (72.4%) 69 (48.9%) <0.001 No 42 (27.6%) 71 (51.1%) Phase I 20 (13%) 25 (17.2%) 0.409 II 101 (65.6%) 97 (66.9%) III 32 (20.8%) 21 (14.5%) IV 1 (0.6%) 2 (1.4%) Evidence Level 1 0 (0%) 1 (0.7%) 0.005 2 76 (49.4%) 52 (35.9%) 3 78 (50.6%) 87 (60%) 4 0 (0%) 5 (3.4%) Experimental Yes 35 (47.3%) 26 (50%) 0.765 Superiority No 39 (52.7%) 26 (50%) Open Access Yes 93 (60.8%) 61 (43.9%) 0.004 Article No 60 (39.2%) 78 (56.1%)
8eea62084ca7e541d918e823422bd82e Conclusion
Studies funded by pharmaceutical industry had stronger evidence, tested more innovative therapies, and were more accessible to the readers compared with studies developed with other sources of funding. These findings may alert oncology cooperative groups to the need of more studies with more evidence strength.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-68 - Correlation of the Lung Immune Prognostic Index (LIPI) and PDL1 Status with Outcomes for Immune Checkpoint Inhibitors in Advanced NSCLC Patients (ID 14256)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
Baseline LIPI, based on derived NLR (neutrophils/[leucocytes-neutrophils]) and lactate dehydrogenase (LDH) was associated with outcomes for immune checkpoint inhibitors in advanced NSCLC patients. We assessed the correlation between LIPI and PDL1 for ICI outcomes in NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
Baseline dNLR and LDH and clinical data were retrospectively collected in advanced NSCLC patients, treated with PD1/PDL1 +/- CTLA4 inhibitors from Nov. 2012 to Mar. 2018, in a multicentric cohort (N=794) from 11 centers. LIPI stratified 3 groups: good (dNLR<3+LDH<upper limit of normal (ULN), intermediate (dNLR>3 or LDH>ULN), poor risk (dNLR>3+LDH>ULN). PDL1 positivity was defined as ≥ 1% tumor cells expression by immunohistochemistry.
4c3880bb027f159e801041b1021e88e8 Result
476 patients (60%) were male, 693 (87%) smokers, 695 (88%) had PS ≤1, with median age 65; 576 (73%) had nonsquamous histology. PDL1 was ≥ 1% in 195 (70%) patients, negative in 82 (30%), and unknown in 517. The median of prior lines was 1 (0-11). The median PFS and OS were 4 months (m) [95% CI 4-5] and 12 m [10-15]. dNLR was>3 in 276 (35%) and LDH>ULN in 290 (37%) patients. LIPI stratified 349 patients as good (44%), 323 (41%) as intermediate and 121 (15%) as poor LIPI risk groups. LIPI was an independent factor for OS (table) and PFS (HR 2.58; CI 1.3-5.2, P=0.02). ≥ 1% PDL1 and ≥ 50% PDL1 were not correlated with OS and PFS. Median OS for good, intermediate, and poor LIPI risk groups were 21 m [17-23], 11 m [9-14] and 4 m [2-6], respectively (P=<0.0001). Median PFS for good, intermediate, and poor risk was 5 m [5-7], 4 m [3-5], and 2 m [1-3], respectively (P=0.0005). No differences were observed in LIPI groups according to the PDL1 expression.
8eea62084ca7e541d918e823422bd82e ConclusionMultivariate analysis for OS
HR
95% CI
P value
Immunotherapy line
>2
2.117
0.641
6.992
0.219
N# Metastasis sites
≥2
1.242
0.727
2.121
0.428
Performance status
≥2
2.141
1.059
4.332
0.034
Albumin
>35 g/dL
0.867
0.507
1.48
0.6
LIPI
Intermediate
Poor
1.697
4.178
0.917
1.956
3.142
8.925
0.001
PDL1 IHC
≥1%
0.713
0.406
1.252
0.239
Baseline LIPI is associated with ICI outcomes in advanced NSCLC, regardless the PDL1 expression. LIPI should be evaluated in prospective clinical trials.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.04 - Immunooncology (Not CME Accredited Session) (ID 936)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.04-19 - Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Predict Survival After Immunotherapy in Non-Small Cell Lung Cancer (ID 13650)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
Effective use of immune checkpoint inhibitors in non-small cell lung cancer (NSCLC) may be improved by identifying biomarkers that are easily measured and predictive of clinical outcomes. Peripheral complete blood counts are commonly obtained and can be indicative of systemic inflammatory response, which has been associated with poor prognosis in multiple cancer types. Here, we investigated the ability of peripheral cell counts to predict patient survival after treatment with immunotherapy for NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
Complete blood counts were retrospectively collected for 274 patients and analyzed for absolute neutrophil count (ANC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR). Values were obtained immediately prior to treatment initiation. Overall survival (OS) and progression-free survival (PFS) was assessed using Kaplan-Meier analyses with log-rank test and Cox regression analysis.
4c3880bb027f159e801041b1021e88e8 Result
OS was significantly associated with ANC (HR 1.63, p<0.0001) and NLR>5 (HR 1.62, p<0.0001), as was PFS (HR 1.48, p=0.0008 and HR 1.48, p=0.0012, respectively). PLR>400 was not associated with OS but did have a significant association with PFS (HR 1.39, p=0.0388). Baseline elevation of both NLR and PLR identified a particularly high-risk population, with worse OS (HR 1.69, p=0.0020) and PFS (HR 1.58, p=0.0069) when compared to the patient group with low baseline NLR and PLR (Figure 1). High NLR and PLR as a combined marker remained an independent predictor of OS in multivariate analysis after adjusting for multiple clinical variables (HR 1.91, p=0.008).
8eea62084ca7e541d918e823422bd82e Conclusion
Increased baseline ANC, NLR, and PLR were associated with worse overall and progression-free survival, and the combination of both high NLR and PLR denoted a subgroup with especially poor outcomes. These findings will need to be validated in larger prospective studies to further assess their clinical utility.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 947)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.15-10 - Comparison of Selected Colombian National Administrative Cancer Registry (NACR) Data in Lung Cancer with the U.S. (ID 12487)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
Lung cancer (LC) is the seventh leading cause of cancer in Colombia and the leading cause of cancer mortality in the United States. In Colombia, previous estimates of lung cancer have been limited to city specific cancer registry (Cali Cancer Registry) and broad estimates from GLOBOCAN. The Colombian Health Ministry in 2015 created the National Administrative Cancer Registry (NACR) to obtain national cancer data. We investigated selected 2016 NACR data and compared it to data from the U.S. to gauge for areas of improvement in lung cancer delivery.
a9ded1e5ce5d75814730bb4caaf49419 Method
We obtained NACR data compiled from the Colombian Department of Health Ministry regarding lung cancer from 2015-2016. We compared this to U.S. data consisting of Surveillance, Epidemiology, and End Results (SEER) and National Cancer Database (NCDB) data from 2003-2015.
4c3880bb027f159e801041b1021e88e8 Result
NACR data in 2016 shows incidence of 1.7 cases per 100,000 and mortality of 2.5 cases per 100,000. SEER data from 2015 shows incidence of 47.2 cases per 100,000 and mortality of 40.6 cases per 100,000. NACR data shows average age of 66 (IQR = 65.7-66) while NCDB data shows median age to be in the age 60-69 category. NACR data in 2016 shows that 14.02% of 849 diagnosed LC patients received surgery compared to 24.83% of 168,093 diagnosed non-small cell lung cancer and small cell lung cancer patients in 2015 from the NCDB. NACR data demonstrates that of those receiving chemotherapy with documented regimens (n=275), 52% received carboplatin, 28.3% received pemetrexed, 27.6% received cisplatin, 25.4% received paclitaxel, 10.9% received bevacizumab, and 5.1% received erlotinib. In comparison, SEER data from 2000-2011 show metastatic NSCLC patients receiving antineoplastic agents (n = 2022) with increasing use of pemetrexed (39.2%), erlotinib (20.3%), and bevacizumab (18.9%) and declining use of paclitaxel (38.7%), gemcitabine (17.0%), and vinorelbine (5.7%). NACR data shows median wait time from diagnosis to first treatment of 31 days (IQR=14-62, n=346) compared to NCDB data showing median wait time of 35 days from diagnosis to first treatment.
8eea62084ca7e541d918e823422bd82e Conclusion
Data shows significantly higher incidence and mortality in the U.S.; this is multifactorial due to screening and data reporting. Comparison shows higher rates of surgery and use of biologics in the U.S. There were similar wait times from diagnosis to first treatment and age of LC patients. Limitations include limited reporting on chemotherapy, radiation, and staging. Future improvements from a Colombian standpoint will include outcomes data collection, increased screening, resources for surgery, and updated access to antineoplastic agents.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.15-26 - A Review of Colombian National Administrative Cancer Registry (NACR) Data to Evaluate Healthcare Delivery and Biologics Use (ID 14354)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
The Office of High Cost of the Colombian Health Ministry created the National Administrative Cancer Registry (NACR) data first in 2015 to provide comprehensive cancer data to improve cancer outcomes while serving as a model for other resource-limited countries. Despite new targeted therapies throughout the world, the benefits of these therapies have not reciprocated in lower resource settings, notably in Latin America. The purpose of this study is to investigate aspects of the NACR data that underscore some of the health care limitations of lung cancer treatment in Colombia.
a9ded1e5ce5d75814730bb4caaf49419 Method
We obtained National Administrative Cancer Registry (NACR) data from the High-Cost Diseases Office (Cuenta de Alto Costo [CAC]) collected in 2015 and released in 2016. All cancer cases diagnosed in the country are reported by payers and providers otherwise there are no payments for services rendered, assuring that the registry is representative. We use descriptive statistics for presentation of data and comparisons.
4c3880bb027f159e801041b1021e88e8 Result
A total of 3,082 patients were analyzed of which 2,043 (66.29%) had contributive insurance, 820 (26.60%) had subsidized insurance, and 98 (3.18%) had special or exempt insurance. Four patients (0.12%) had no insurance. Of newly diagnosed patients, the median number of days from suspicion to diagnosis was 27 days (IQR = 12-45 days, n = 491) with the predominant range of patients with contributive insurance being 30-59 days, and for subsidized insurance being 15-29 days. The median number of days from diagnosis to first treatment was 31 days (IQR=14-62, n=346) with the predominant range for patients with both contributive and subsidized insurance being 30-59 days. There was a greater percentage of Stage IV cancers in patients with subsidized (34%) than contributive (23%) insurance. Of those receiving chemotherapy (n=275), 52% received carboplatin, 28.3% received pemetrexed, 27.6% received cisplatin, 25.4% received paclitaxel, 10.9% received bevacizumab, and 5.1% received erlotinib; no patients received nivolumab or pembrolizumab.
8eea62084ca7e541d918e823422bd82e Conclusion
Based on findings from NACR, the wait time from suspicion to treatment took nearly two months underscoring the need for better streamline of lung cancer care. Also, data shows a low percentage of use of newer therapies, including EGFR-targeted agents despite a high prevalence of mutations, which are present in around a quarter of patients in Colombia (Raez, 2017). Colombia can strongly benefit from increased access to molecular testing and biologics given the future direction of lung cancer therapy.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-82 - Neutrophil-to-Lymphocyte Ratio Complements the Prognostic Ability of PD-L1 in Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors (ID 14091)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
PD-L1 expression is an imperfect predictor of outcomes for patients (pts) with advanced non-small cell lung cancer (aNSCLC) treated with PD-1/PD-L1 inhibitors (PD-1/L1i). This was demonstrated in the recent ARCTIC trial in which PD-L1 expression did not significantly correlate with outcomes. In the quest for additional markers, a high neutrophil-to-lymphocyte ratio (NLR) has been associated with poor outcomes and may reflect a higher myeloid-to-lymphoid balance. Here we show improved prognostic ability for response to PD-1/L1i when baseline NLR is added to PD-L1 expression.
a9ded1e5ce5d75814730bb4caaf49419 Method
We used a retrospective cohort of 146 aNSCLC pts from the authors’ institutions in the United States and Japan who received single-agent PD-1/L1i. We categorized patients into three groups; favorable: PD-L1 ≥ 1% and NLR < 5, intermediate: PD-L1 ≥ 1% or NLR < 5, and poor: PD-L1 < 1% and NLR ≥ 5. We correlated the outcome of each group with overall survival (OS) and progression free survival (PFS).
4c3880bb027f159e801041b1021e88e8 Result
Median follow-up was 11.1 months (M) (95% Confidence Interval [CI]: 9.1-13.1). 47 pts had PD-L1 <1% and 99 pts ≥1%, 81 pts had NLR <5 and 65 pts ≥5. There were 52, 76, and 18 pts in the favorable, intermediate, and poor groups, respectively. Median OS for the favorable group was not reached and it was 14.7 M (CI: 10.5-19.0) and 3.5 M (CI: 0-13.1), respectively for the intermediate and poor groups. Median PFS was 9.9 M (CI: 3.7-16), 3.2 M (CI: 2.1-4.3), and 1.1 M (CI: 0.8-1.4), respectively. The poor group (PD-L1 < 1% and NLR ≥ 5) was significantly associated with progressive disease (Odds ratio [OR]: 5.0, p=0.01) in comparison to the PD-L1 < 1% group (OR: 2.6, p=0.013).
8eea62084ca7e541d918e823422bd82e Conclusion
Prognostic ability of PD-L1 expression is enhanced when combined with baseline NLR for aNSCLC pts treated with single-agent PD-1/L1i. This study raises the hypothesis that high NLR and low PD-L1 expression could serve to identify those pts less likely to benefit from these therapies.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 964)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 3
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.15-16 - Clinical Economic Impact of Improved Genotyping in Patients with Advanced Non-Small Cell Lung Adenocarcinoma (NSCLC) (ID 14255)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
Comprehensive genomic profiling (CGP) at diagnosis and progression identifies NSCLC patients who may benefit from targeted therapies and are unlikely to respond to immunotherapy, however many patients are incompletely or undergenotyped. We developed a cost benefit model to evaluate the clinical and economic impact of using plasma-based cfDNA CGP to guide treatment decisions in first- and second-line advanced NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
The model compares the clinical and economic impact of an NCCN guideline driven care paradigm, utilizing Guardant360â (G360), a CLIA certified, CAP accredited, NYSDOH approved cfDNA CGP test, for stage IIIB/IV NSCLC patients versus the current care paradigm and assesses the impact of additional genomic information to aid in therapy selection and subsequent effects on biopsy rates, drug costs, and clinical outcomes (RR, PFS, and median OS). The model targeted patients with NSCLC receiving first or second line treatment enrolled in a U.S. Commercial Health Plan with 10 million lives. Frequency of NCCN genomic targets in first-line patients was per The Cancer Genome Atlas with second-line frequencies modified to reflect the first-line testing, genotyping QNS, biopsy, and undergenotyping rates. Therapy current care distributions were derived from 2017 Integra Connect’s proprietary database.
4c3880bb027f159e801041b1021e88e8 Result
Under the guideline directed care, immunotherapy and chemotherapy use decreased as patients are re-assigned to targeted therapy in both first line QNS and second line progression settings, resulting in improved clinical outcomes, including a second line repeat tissue biopsy rate reduction. Individual and overall cost savings were observed in both settings (Table).
8eea62084ca7e541d918e823422bd82e ConclusionFirst-Line QNS Patients
Second-Line Patients
Current
Guideline Directed
Difference
Current
Guideline Directed
Difference
Overall Patient Cohort
Immunotherapy Monotherapy
19.2%
13.2%
- 6.00%
52.5%
42.9%
- 9.6%
Immunotherapy + Chemotherapy
5.6%
3.9%
- 1.7%
3.3%
2.7%
- 0.6%
Chemotherapy +/- Biologics
75.2%
51.7%
- 23.5%
35.7%
29.2%
- 6.5%
Targeted Therapy
0%
31.3%
+ 31.3%
8.6%
25.3%
+ 16.7%
Overall Clinical Outcome Measures
Response Rate
18.1%
28.3%
+ 10.2%
20.9%
25.7%
+ 4.8%
Progression Free Survival (months)
4.7
6.0
+ 1.3
4.7
5.6
+ 0.9
Overall Survival (months)
11.1
12.6
+ 1.5
11.0
12.1
+ 1.1
Reassigned Patient Cohort
Immunotherapy Monotherapy
6.0%
-
-6.0%
9.6%
-
- 9.6%
Immunotherapy + Chemotherapy
1.8%
-
- 1.8%
0.6%%
-
- 0.6%
Chemotherapy +/- Biologics
23.5%
-
- 23.5%
6.5%
-
- 6.5%
Targeted Therapy
-
31.3%
+ 31.3%
-
16.7%
+ 16.7%
Clinical Outcome Measures for Reassigned Patients
Response Rate
18.1%
50.7%
+ 32.6%
17.7%
49.5%
+ 31.8%
Progression Free Survival (months)
4.7
8.8
+ 4.0
4.2
9.7
+ 5.5
Overall Survival (months)
11.1
15.9
+ 4.9
10.3
17.3
+ 7.0
Second-line re-biopsy rate
-
-
-
25%
11%
-14%
Overall Cost per Patient
$140,950
$130,141
-$10,809
$175,563
$161,810
- $13,753
Overall Total Cost
$55,516,110
$51,258,713
-$4,257,397
$209,553,002
$193,136,843
- $16,416,159
cfDNA CGP application in first line tissue QNS and second line progressing advanced NSCLC patients improved outcomes and cost savings. Shifting from chemotherapy and immunotherapy to relatively more efficacious, less toxic, and less expensive targeted therapies resulted in improved patient outcomes. Cost savings are driven by decrease in immunotherapy, infusion costs, emergency room/hospital visits, and avoidance of tissue biopsy.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.15-18 - Cost-Effectiveness Analysis of Second-Line Immune Checkpoint Inhibitors for Advanced NSCLC in Colombia (ID 14109)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
Immune checkpoint inhibitors improve outcomes compared with chemotherapy in lung cancer. Tumor PD-L1 receptor expression is being studied as a predictive biomarker. The greatest challenge in oncology today is how to reconcile improvements in the management of cancer with the exponentially increasing costs of new treatment and this is a very important barrier in low and middle income countries. The objective of this study was to assess the cost-effectiveness and economic impact of second-line treatment with nivolumab and pembrolizumab with and without the use of PD-L1 testing for patient selection in Colombia .
a9ded1e5ce5d75814730bb4caaf49419 Method
We designed a decision-analytic model to evaluate the cost-effectiveness of second-line immunotherapy versus docetaxel for advanced NSCLC. We considered the outcomes from randomized clinical trials (RCTs). Direct and indirect costs were retrieved with a colombian perspective. We followed standard recommendations for the conduct and reporting of health economic analyses. Primary endpoint were Incremental Cost Effectiveness Ratios (ICER), for treatment with nivolumab and pembrolizumab with and without PDL1 testing.
4c3880bb027f159e801041b1021e88e8 Result
Nivolumab improved quality-adjusted life-years (QALY) by 0.417 among squamous tumors and 0.287 among non squamous tumors. The ICER were $135,093 COP and $179,391 COP, respectively. Pembrolizumab achieved a QALY gain of 0.346 and the ICER was $146,022 COP. The use of PD-L1 expression as a tailor biomarker for nivolumab among non-squamous tumors improved incremental QALY by up to 157% and decreased the ICER by up to 61% compared with treating all patients. Considering a willingness to pay threshold of three times the Colombian Gross Domestic Product per capita, second-line immunotherapy was not cost-effective with or without patient selection by PD-L1 expression.
8eea62084ca7e541d918e823422bd82e Conclusion
Patient selection by PD-L1 expression increased cost-effectiveness of immunotherapy. Second-line immunotherapy was not cost-effective in Colombia due to its high cost. Taking into account the disparities in access to cancer innovative therapies, there is a need to promote strategies to reduce drug acquisition costs, such as price discrimination and the use of biosimilars or generics.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.15-23 - Are there Ethnic Disparities in the Clinical Outcomes of Non-Small Cell Lung Cancer Hispanic Patients Treated with Immunotherapy? (ID 12359)
16:45 - 18:00 | Author(s): Gilberto de Lima Lopes
- Abstract
Background
Immunotherapy outcomes in non-small cell lung cancer (NSCLC) are widely available thanks to studies that got the approval of PD-1/PD-L1 inhibitors. However a careful review of ethnicity can find that most of the studies were done in Non-Hispanic White or Asian populations. There is little known about the outcomes in Hispanics (H). It is well known that Hispanics (H) in the US seem to have a lower age-adjusted mortality in NSCLC and have a different gene expression profile than NHW with higher prevalence of EGFR mutations.
a9ded1e5ce5d75814730bb4caaf49419 Method
We reviewed clinical outcomes in 216 H pts with NSCLC stage IV treated with atezolizumab, nivolumab or pembrolizumab at 4 large cancer centers (Memorial Cancer Institute, University of Miami and Moffitt Cancer Center all of them in Florida (US), and the National Cancer Institute in Peru. These patients have failed at least one line of chemotherapy previously. All of these patients did not have actionable genes (EGFR. ALK, ROS-1). We assessed overall response rate ORR (CR+PR) as main objective and disease control rate (DCR: ORR+SD), median PFS (progression free survival) & overall survival (OS) and PFS at 6m and 12m as secondary objectives.
4c3880bb027f159e801041b1021e88e8 Result
Most of the pts were males: 116 (54%), 82% adenocarcinomas and the median age was 65 years (range: 37-88y). The ORR was 16% and the DCR that shows the clinical benefit was 67%. ORR and DCR were similar in adenocarcinomas (20%/68%) and squamous cell carcinomas (17%/64%). The progression free survival (PFS) at 6 months (m) and 12m were 80% and 56% respectively. Median PFS 14.5m and median overall survival were 19m, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
ORR for NSCLC pts treated with immunotherapy is 16% in Hispanics treated at 4 cancer centers compared to an expected 20% ORR for NHW as reported in the literature. Therefore it appears that Hispanics might not have a benefit from immunotherapy to the extent that NHWs do. We need a larger cohort and prospective studies to validate these findings.
6f8b794f3246b0c1e1780bb4d4d5dc53


