Virtual Library
Start Your Search
Yi-Long Wu
Author of
-
+
JCSE01 - Perspectives for Lung Cancer Early Detection (ID 779)
- Event: WCLC 2018
- Type: Joint IASLC/CSCO/CAALC Session
- Track: Screening and Early Detection
- Presentations: 3
- Moderators:
- Coordinates: 9/23/2018, 07:30 - 11:15, Room 202 BD
-
+
JCSE01.09 - Cluster Trial: Ph2 Biomarker-Integrated Study of Single Agent Alpelisib, Capmatinib, Ceritinib and Binimetinib in advNSCLC (ID 11678)
10:15 - 10:25 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
Several genetically altered signaling pathways have been profiled in NSCLC, enabling advanced management of NSCLC using targeted therapies. This study investigated the therapeutic spectrum of NSCLC with uncommon molecular alterations by allocating patients to treatment arms based on molecular aberrations; targeted therapies alpelisib (PI3Kαi), capmatinib (METi), ceritinib (ALKi), and binimetinib (MEKi) were evaluated.The study was based on the umbrella design. Key objectives: investigate feasibility of using one trial for different agents based on biomarker-integrated analysis, assess anti-tumor activity, characterize safety, tolerability and PK profiles of individual agents. Key eligibility criteria: age ≥18 years; ECOG PS ≤2; failed prior treatment/unsuitable for chemotherapy. Documentation of locally determined molecular alterations before treatment allocation was required (alpelisib, 350 mg QD: PIK3CA mutation/amplification; capmatinib, 400 mg BID (tablet): MET IHC overexpression/amplification; ceritinib, 750 mg QD: ALK or ROS1 rearrangement; binimetinib, 45 mg BID: KRAS, NRAS or BRAF mutation).Sixty-six patients with advNSCLC were enrolled (median age 58 years; 65.2% male: alpelisib, n=2; capmatinib, n=16; ceritinib, n=26; binimetinib, n=22). As of Feb 28, 2018, 10 patients in ceritinib and 2 in binimetinib arms were ongoing. Twenty-four patients had confirmed partial responses (36.4%): alpelisib, 0%; capmatinib, 18.8%; ceritinib, 73.1%; binimetinib, 9.1% (Figure). Longest mPFS (14.4 months) was in ceritinib arm. Among the most common treatment-related AEs: alpelisib: malaise, hyperglycemia, dysgeusia; capmatinib: nausea, anemia, peripheral edema, decreased appetite; ceritinib: diarrhea, vomiting, ALT/AST elevation; binimetinib: mouth ulceration, AST, blood CPK increased, rash. Most AEs were grade 1/2.
Objective responses/tumor shrinkage were observed in the study; highest ORR and mPFS were observed with ceritinib, although patient numbers differed between arms. All treatments were well tolerated; no new safety signals were observed. This study demonstrated the feasibility of an umbrella trial and importance of precision medicine in the management of advNSCLC with uncommon molecular alterations.
a9ded1e5ce5d75814730bb4caaf49419Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
JCSE01.18 - A Multicenter Survey of One Year Survival Among Chinese Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer (CTONG1506) (ID 14707)
11:15 - 11:15 | Author(s): Yi-Long Wu
- Abstract
Background
Previous results of CTONG1506 study showed that gene aberration test rate was increasing in Chinese NSCLC patients and first-line treatment was standardized accordingly. This survey further described one year survival of patients with different gene aberration status and under different first-line treatments.CTONG1506 was a two-year series cross-sectional study. Patients with advanced nonsquamous NSCLC who were admitted from August 2015 to March 2016 and who received first-line anti-cancer treatment at one of 12 tertiary hospitals across China were included. Data extracted from medical charts were entered into medical record abstraction forms, which were collated for analysis. Survival information was collected one year after patients were admitted to hospital. One year survival rate and its 95% confidence interval were analysed by Kaplan-Meier method.
A total of 707 patients were analysed, with mean age of 57 years and 56.7% were male. Among the 487 patients who had survival data, 192 were EGFR- mutation positive (86 mutated in exon 19 [one year survival rate 0.90, 95% CI: 0.81-0.94] and 88 mutated in exon 21 [one year survival rate 0.84, 95% CI: 0.75-0.90]), 27 patients were ALK positive and 164 patients were EGFR and ALK wild type. Most EGFRmutation positive patients (128/192) received tyrosine kinase inhibitors (TKIs) as first-line treatment and most EGFR wild type patients (155/175) received first-line chemotherapy (Chemo). Pemetrexed was the most common non-platinum chemotherapy-backbone agent (120/155) in platinum doublet regimens. One year survival rates are shown in the table.
This national-wide real world study of tertiary hospitals in China revealed that a majority of (>75%) advanced nonsquamous NSCLC patients survived more than one year and was comparable to well-controlled clinical trial results, indicating survival benefits by gene aberration status guided standard of care. This result may be further validated by our on-going two-year survey.
a9ded1e5ce5d75814730bb4caaf49419 -
+
JCSE01.22 - Differential Molecular Mechanisms Associated with Dramatic and Gradual Progression in NSCLC Patients with Intrathoracic Dissemination (ID 14713)
11:15 - 11:15 | Author(s): Yi-Long Wu
- Abstract
Background
Lung cancer is a highly heterogeneous disease with diverse clinical outcomes. The pleural cavity is a frequent metastasis site of proximal lung cancer. Better understanding of its underlining molecular mechanisms associated with dramatic and gradual progression of pleural metastasis in patients with non-small cell lung cancer (NSCLC) is essential for prognosis, intervention and new therapy development.We performed whole-exome sequencing (WES) of matched primary lung adenocarcinoma and pleural metastatic tumors from 26 lung cancer patients with dramatic progression (DP, n=13) or gradual progression (GP, n=13). Somatic alterations at both genome-wide level and gene level were detected. Kaplan-Meier survival analysis and multivariate Cox regression models were applied to analyze the association between different somatic alterations and clinical parameters.We first analyzed the differences in somatic alterations between AP and RP group in the primary tumors, and identified higher somatic copy number alteration (SCNA) level in DP group compared to GP group, which is significantly (p=0.016) associated with poorer progression-free survival (PFS). More specifically, patients with chromosome 18q loss in the primary tumor showed a trend (p=0.107) towards poorer PFS. PTEN (p=0.002) and GNAS (p=0.002) mutations are enriched in the primary tumors of DP group, and are associated with poorer PFS. Furthermore, pleural metastatic tumors harbor a relatively higher level of mutation burden (p=0.105) and significantly increased SCNA (p=0.035) compared to the primary tumors.NSCLC patients in the attenuated progression group have more stable genomes. High level of genomic instability, GNAS and PTENmutations, as well as chromosome 18q loss are associated with rapid progression. a9ded1e5ce5d75814730bb4caaf49419
-
+
MA02 - Improving Outcomes for Patients with Lung Cancer (ID 895)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 201 BD
-
+
MA02.03 - ASTRIS: A Real World Treatment Study of Osimertinib in Patients with EGFR T790M-Positive NSCLC (ID 12972)
10:40 - 10:45 | Presenting Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
Osimertinib is a third-generation, CNS-active EGFR-TKI that potently and selectively inhibits both EGFR-TKI sensitizing and EGFR T790M resistance mutations. We report results from a second planned protocol, optimal interim analysis of the ongoing ASTRIS study (NCT02474355).
a9ded1e5ce5d75814730bb4caaf49419 Method
Eligible patients receive osimertinib 80 mg once daily. Inclusion criteria: stage IIIB/IV T790M-positive non-small cell lung cancer (NSCLC); T790M status confirmed locally by validated test, not restricted by sample type; prior EGFR-TKI therapy received; WHO performance status (PS) 0−2; acceptable organ and bone marrow function and no history of interstitial lung disease (ILD) or QTc prolongation. Asymptomatic, stable CNS metastases are permitted. The primary efficacy outcome is overall survival (OS).
4c3880bb027f159e801041b1021e88e8 Result
From Sept 18, 2015, first patient in, to Oct 20 2017 data cut-off (DCO), 3014 patients were enrolled across 16 countries and received ≥1 dose of osimertinib (full analysis set [FAS]): median follow-up 7.9 months (range <1−24), median age 62 yrs (27–92), 64% female, 69% Asian, 30% White, 11% WHO PS 2, 45% prior chemotherapy, 34% prior radiotherapy. All patients had T790M-positive status, identified from tissue in 1610 patients (53%), plasma ctDNA in 1241 patients (41%) and from other sources in 162 patients (5%). At DCO, 1276 patients (42%) had discontinued treatment (1738 [58%] ongoing); median duration of exposure 7.4 months (<1–25); 1289 patients (43%) had a progression-free survival (PFS) event, 1276 (42%) had a time to treatment discontinuation (TTD) event, and 593 (20%) had died. In patients evaluable for response, the investigator-assessed clinical response rate was 56.6% (1625/2872; 95% confidence interval [CI] 54.7, 58.4). In the FAS, estimated median PFS was 11.0 months (95% CI 10.6, 11.1), median TTD was 12.6 months (95% CI 12.2, 13.7), and median OS was not reached (OS at 12 months was 75.8% (95% CI 73.7, 77.8). Adverse events (AEs) leading to dose modification and treatment discontinuation were reported in 321 patients (11%) and 147 patients (5%), respectively. Serious AEs were reported in 505 patients (17%). ILD/pneumonitis-like events were reported in 41 patients (1%), and QTc prolongation in 48 patients (2%).
8eea62084ca7e541d918e823422bd82e Conclusion
ASTRIS, the largest reported study of osimertinib in T790M-positive NSCLC, demonstrates clinical activity similar to that observed in the osimertinib clinical trial program with no new safety signals.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15 - Colliding Approaches - EGFR and Immunotherapy (ID 916)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 107
-
+
MA15.01 - Strong PD-L1 Expression Predicts Poor Response and de Novo Resistance to EGFR TKIs Among Non-Small Cell Lung Cancer Patients with EGFR Mutation (ID 12920)
13:30 - 13:35 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
This study evaluated whether tumor expression of programmed death-ligand 1 (PD-L1) predicted the response of EGFR-mutated non-small cell lung cancer (NSCLC) to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs).
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively evaluated patients who received EGFR-TKIs for advanced NSCLC between April 2016 and September 2017 at the Guangdong Lung Cancer Institute. None of them were enrolled in clinical studies, and their EGFR and PD-L1 statuses were simultaneously evaluated.
4c3880bb027f159e801041b1021e88e8 Result
Among the 101 eligible patients, strong PD-L1 expression significantly decreased the objective response rate (ORR) compared with those with weak or negative PD-L1 expression (35.7% vs 63.2% vs 67.3%, P=0.002) as well as shortened progression-free survival (PFS, 3.8months vs 6.0months vs 9.5months, P<0.001), regardless of EGFR mutation types (19del or L858R). Furthermore, positive PD-L1 expression was predominantly observed among patients with de novo resistance rather than acquired resistance to EGFR-TKIs (66.7% vs 30.2%, P=0.009). Notably, patients with de novo resistance had a high proportion of dual positive for PD-L1 and CD8 (46.7%, 7/15). Finally, one patient with de novo resistance to EGFR-TKIs and dual positivity for PD-L1 and CD8 experienced a favorable response to anti-PD-1 therapy.
8eea62084ca7e541d918e823422bd82e Conclusion
This study uncovered the adverse impact of PD-L1 expression on the efficacy of EGFR- TKIs, especially in those with de novo resistance NSCLC, which inclined to reshape an inflamed immune phenotype of dual positive for PD-L1 and CD8 and showed potential therapeutic sensitivity to PD-1 blockade.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15.06 - Circulating Tumor DNA Portrays the Resistance Landscape to a Novel Third Generation EGFR Inhibitor, AC0010 (ID 13641)
14:05 - 14:10 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
In a Phase I/II dose-escalation and expansion study conducted at Guangdong Lung Cancer Institute, AC0010 demonstrated promising efficacy and good tolerability in advanced NSCLC patients with EGFR T790M-mediated resistance to previous EGFR TKIs, (NCT02330367). As disease progression (PD) with EGFR T790M-directed therapy also emerges over time, we investigated the resistance mechanisms to AC0010 in this study.
a9ded1e5ce5d75814730bb4caaf49419 Method
Serial ctDNA samples obtained from patients who developed PD with AC0010 were analyzed using ultra-deep sequencing capturing 295 cancer-related genes. Alterations that were absent before treatment and acquired at PD or that increased in abundance during treatment were identified as putative resistance mechanisms.
4c3880bb027f159e801041b1021e88e8 Result
Longitudinal plasma samples were obtained from 23 patients who progressed on AC0010 (data cut-off October 14, 2016; figure1). Putative resistance mechanisms to AC0010 were identified in 19/23 patients (>1 putative resistance mechanism was detected in some patients). EGFR amplification was the predominant resistance mechanism (21.1% [4/19 patients]), followed by TP53 loss of heterozygosity (15.8% [3/19]). EGFR C797S mutation, Met amplification and mutations in the PI3KCA pathway each occurred in 10.5% of patients (2/19). SCLC transformation, ERBB2 amplification, CD79A_A32G mutation, CDKN2A_R80 mutation, CRLF2 amplification, MLH1 amplification, Rb1 loss, and concurrent rise in the allelic fraction of tumor suppressor gene TP53 and Rb1 were each detected in 5.3% of patients (1/19). In a patient with PD following single-agent AC0010 and EGFR amplification as the putative resistance mechanism to AC0010, subsequent treatment with AC0010 plus nimotuzumab (EGFR monoclonal antibody) successfully overcame resistance, resulting in a response that lasted for 7.7 months.
8eea62084ca7e541d918e823422bd82e Conclusion
The resistance landscape to AC0010 appears to differ from that described previously with osimertinib. In this cohort of patients in China, EGFR amplification was the predominant resistance mechanism to AC0010 and could be potentially overcome by EGFR dual inhibition.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA16 - Novel Mechanisms for Molecular Profiling (ID 917)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Advanced NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 203 BD
-
+
MA16.10 - Clinical Utility of Cerebrospinal Fluid Cell-Free DNA for Clarifying Genetic Features of Leptomeningeal Metastases in ALK Rearrangement NSCLC (ID 12142)
14:35 - 14:40 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
Leptomeningeal metastases (LM) were associated with a poor prognosis in non small cell lung cancer (NSCLC). LM were much more frequent in EGFR mutant patients, and cerebrospinal fluid (CSF) cell-free DNA (cfDNA) has shown unique genetic profiles of LM in patients harboring EGFR mutations in our previous studies. However, studies in ALK positive NSCLC patients with LM are scarce.
a9ded1e5ce5d75814730bb4caaf49419 Method
Lung cancer patients with ALK rearrangement were screened from Sept 2011 to Feb 2018 at our institute. Leptomeningeal metastases were diagnosed by MRI or CSF cytology or next-generation sequencing (NGS) of CSF cfDNAs. Paired plasma were also tested by NGS.
4c3880bb027f159e801041b1021e88e8 Result
LM were diagnosed in 22 (7.6%) of 288 ALK rearrangement patients with lung cancer. A total of 11 ALK positive patients with LM were enrolled with CSF cfDNA tested by NGS (one case used CSF precipitates instead of CSF cfDNA). Paired plasma were available in 11 patients. Driver genes were detected in 75.0% CSF samples and 45.5% plasma respectively (P=0.214). Max allele fractions were higher in CSF cfDNA than in plasma (40.8% versus 0%, P=0.021). ALK variant 1 (E13:A20) was detected in 3 cases of CSF and paired plasma, respectively. ALK variant 2 (E20:A20) was identified in 5 cases of CSF and 1 paired plasma. Multiple copy number variants (CNV) were mainly found in CSF cfDNA, including EGFR copy number gains. Resistance mutations including gatekeeper gene ALK G1202R was identified in CSF cfDNA with ALK variant 1 and ALK G1269A was detected in plasma. The detection rate of TP53 was 45.4% versus 27.3% in CSF cfDNA and plasma.
8eea62084ca7e541d918e823422bd82e Conclusion
CSF cfDNA was more sensitive than plasma to reveal genetic features of ALK-fusion LM, confirming its role as a liquid biopsy medium for LM in driver gene positive NSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA24 - Genomic Evolution, KEAP 3 and More Non-Coding RNA (ID 928)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Biology
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 205 BD
-
+
MA24.01 - Genomic Evolution Trajectory Depicts Invasiveness Acquisition from Pre-invasive to Invasive Adenocarcinoma (ID 11840)
10:30 - 10:35 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
Accumulation of molecular abnormalities may depict evolution trajectories of tumor initiation and development. However, the genomic profile of early stage adenocarcinoma and molecular mechanism of invasiveness acquisition from pre-invasive to invasive adenocarcinoma remains barely explored.
a9ded1e5ce5d75814730bb4caaf49419 Method
We simultaneously collected 20 patients with adenocarcinoma in situ (AIS) (n=5), minimally invasive adenocarcinoma (MIA) (n=5) and stage IA adenocarcinoma (lepidic/acinar predominant) (n=10). Whole exon sequencing (WES) was performed in pre-invasive adenocarcinoma with multi-region specimens and stage IA adenocarcinoma. Analysis of genomic alteration among different pathological status was performed and tumor mutation burden (TMB) was calculated as well as six mutation types individually. Enriched pathways of each pathology were measured through KEGG analysis.
4c3880bb027f159e801041b1021e88e8 Result
Baseline characteristics was generated through heatmap with smokers (2/20, 10%) and EGFR mutation (13/20, 65%) among whole population. AIS/MIA indicated much lower number of mutations than invasive adenocarcinoma (IAC) while TMB revealed the same trend without statistical significance. Multi-region sequencing showed high heterogeneity of single nucleotide variation (SNV) in AIS and MIA. Unique SNV presented dominant proportion in initial status. Cluster analysis showed higher copy number variation in AIS/MIA than IAC with cell adhesion molecules (CAMs) enriched in AIS/MIA while variety pathway enrichment in IAC through KEGG analysis. C>A transversions held major proportion in early stage adenocarcinoma and a significant increase in the proportion of C>T and C>G mutation was exhibited when evolving into IAC.
8eea62084ca7e541d918e823422bd82e Conclusion
Intratumor heterogeneity may occur in the very beginning of adenocarcinoma. High copy number variation was dominant event for AIS/MIA while higher tumor mutation burden was seen in IAC. Tobacco signature encompassing C>A transversions dominates the early development of adenocarcinoma and APOBEC signature may play a potential role in acquisition of cancer invasiveness.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA25 - Oligometastasis: Defining, Treating, and Evaluating (ID 929)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Oligometastatic NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 13:30 - 15:00, Room 203 BD
-
+
MA25.06 - RPA Analysis for Oligometastatic Non-Small Cell Lung Cancer: Smoking Combine T3/4 Patients May Not Be Benefit from Local Consolidative Treatment (ID 11994)
14:05 - 14:10 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
In the literature on oligometastasis, the relative importance of local consolidative treatment (LCT) has been gradually accepted. This study set out to investigate the prognosis heterogeneity and the effect of LCT for oligometastatic non-small cell lung cancer patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
We identified 436 patients in Guangdong General Hospital (GGH) from 2009 to 2016 with oligometastatic disease, and the factors predictive of overall survival (OS) were evaluated using Cox regression. Risk stratifications were defined using recursive partitioning analysis (RPA) on training set (2009~2014), which were further confirmed on validation set (2015-2016). And the effect of LCT for different risk groups was further examined by Kaplan-Meier method.
4c3880bb027f159e801041b1021e88e8 Result
Factors predictive of OS were: T stage (p=0.001), N stage (p=0.008), metastatic sites (p=0.031) and EGFR status (p=0.043). Prognostic risk RPA model was established, 4 risk groups were identified: Group I, never smokers and N0 disease (3-year OS: 55.6%, median survival time (MST)=42.8m); Group II, never smokers and N+ disease (3-year OS: 32.8%, MST=26.5m); Group III, smokers and T1/T2 disease (3-year OS: 23.3%, MST=19.4m); and Group IV, smokers and T3/T4 disease (3-year OS: 12.5%, MST=11.1m). Among four groups, OS significant differences were observed according to LCT except group IV (p=0.45).
8eea62084ca7e541d918e823422bd82e Conclusion
This retrospective study identified the poor prognostic population (smoking combine T3/4 disease) of oligometastatic non-small cell lung cancer patients, and this population may not be benefit from local consolidative therapy.

6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA26 - New Therapies and Emerging Data in ALK, EGFR and ROS1 (ID 930)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 13:30 - 15:00, Room 201 BD
-
+
MA26.11 - Effects of Dose Modifications on the Safety and Efficacy of Dacomitinib for EGFR Mutation-Positive NSCLC (ID 13318)
14:40 - 14:45 | Presenting Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
In patients with EGFR mutation-positive advanced stage NSCLC, first-line dacomitinib significantly improved PFS, OS, DoR and time to treatment failure vs gefitinib (ARCHER 1050; NCT01774721).1,2 Dacomitinib starting dose was 45 mg QD for all patients, with reductions to 30 or 15 mg QD permitted. We explored effects of dacomitinib dose reduction on safety and efficacy in this ongoing study.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with newly diagnosed stage IIIB/IV or recurrent NSCLC harboring an EGFR mutation (exon 19 del or exon 21 L858R) randomized to dacomitinib received 45 mg PO QD. Study endpoints and protocol-defined dose reduction parameters were previously described.1 We evaluated reasons for dose reductions, and their effects on incidence and severity of common adverse events (AEs) and key efficacy endpoints (PFS, OS, ORR). Data cutoff dates: 17-Feb-2017 (OS), 29-Jul-2016 (other endpoints).
4c3880bb027f159e801041b1021e88e8 Result
Overall, 150 (66.1%) patients dose reduced for AEs (87 and 63 reduced to 30 and 15 mg QD as lowest dose, respectively); most commonly for skin toxicities (62.6%) and diarrhea (14.0%). Median time to each successive dose reduction was ~12 weeks. Incidence and severity of AEs declined following dose reduction, including grade ≥3 diarrhea (11.3% before vs 4.0% after), dermatitis acneiform (15.3% vs 6.7%), stomatitis (3.3% vs 2.7%) and paronychia (7.3% vs 4.7%).
PFS was similar in dose-reduced and all dacomitinib-treated patients (Figure).

Median OS results were also similar (dose-reduced patients: 36.7 mo [95% CI: 32.6, NR]; all dacomitinib-treated patients: 34.1 mo [95% CI: 29.5, 37.7] as were ORRs (dose-reduced patients: 79.3% [95% CI: 72.0, 85.5]; all dacomitinib-treated patients: 74.9% [95% CI: 68.7, 80.4]).
8eea62084ca7e541d918e823422bd82e Conclusion
Efficacy was similar in the dose-reduced patients and the overall study population. Incidence/severity of dacomitinib-related AEs decreased with dose reduction, thereby allowing patients to continue treatment.
References:
Wu, et al. Lancet Oncol. 2017.
Mok, et al. J Clin Oncol. 2018.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA10 - Right Patient, Right Target & Right Drug - Novel Treatments and Research Partnerships (ID 910)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 10:30 - 12:00, Room 106
-
+
OA10.07 - Resistance Mechanisms of Osimertinib in Chinese Non-Small Cell Lung Cancer Patients: Analysis from AURA17 Trial (ID 12693)
11:35 - 11:45 | Author(s): Yi-Long Wu
- Abstract
- Presentation
Background
Osimertinib is approved for metastatic NSCLC patients with EGFR T790M mutation after progression from TKI therapy. Despite impressive tumor responses, drug resistance usually develops. The resistance mechanisms of osimertinib are emerging but studies with large cohorts of Chinese patients and association with clinical outcomes are lacking. Here we report a biomarker study of osimertinib using plasma samples from 107 Chinese patients who had progressed by 24 months after LSFD (Oct. 2017) of AURA17 (NCT02442349), the 2nd-line pivotal trial in China.
a9ded1e5ce5d75814730bb4caaf49419 Method
Serial plasma cell-free DNA (cfDNA) were collected from baseline until progressive disease (PD) by investigator assessment. Capture-based 75-gene NGS panel with unique molecular index (UMI) system was used to identify resistance mechanisms to osimertinib by comparing paired cfDNA at baseline and PD. Droplet digital PCR (ddPCR) was used to dynamically monitor EGFR mutation changes (L858R, Ex19Del, T790M and C797S) during treatment course. Association of cfDNA biomarkers based on valid test results with objective response rate (ORR), progression-free survival (PFS) and overall survival (OS) from DCO3 (Mar. 20, 2018) was analyzed.
4c3880bb027f159e801041b1021e88e8 Result
The 107 patients were with ORR of 68.2%, median PFS of 8.2 months, and median OS of 21.5 months. Eight-two had detectable EGFR sensitizing mutations (L858R or Ex19Del) in their PD cfDNA samples. Among them, 15 had acquired EGFR C797S, all in cis with T790M, and with no enrichment for L858R or Ex19Del (6 and 9, respectively). The median time of C797S detection from plasma was 2.8 (1.2-8.4) months prior to PD. EGFR L718Q, I744T, C775Y, G796S/D, T854I mutations, or amplification were found in 11 patients. Aberrations in bypass tracks including AKT2, ALK, DDR2, ERBB2/3, HRAS, JAK1/2, KRAS, MET, NTRK1, PIK3CA, RIT1, etc. were observed in 45 patients.
Clearance of EGFR sensitizing mutations at weeks 3 of treatment was associated with favorable ORR (78.7% vs. 33.3%), PFS (9.6 vs. 4.0 months, p<0.001) and OS (21.5 vs. 11.7 months, p<0.001). Clearance of EGFR sensitizing mutations at weeks 6 of treatment was also associated with favorable ORR (80.0% vs. 36.8%) and PFS (8.3 vs. 4.2 months, p<0.001). Presence of T790M at PD was correlated with longer PFS (12.3 vs. 5.5 months, p<0.001) and OS (21.3 vs. 13.2 months, p=0.045). Acquired or enriched TP53 alterations at PD were associated with worse PFS (4.2 vs. 8.3 months, p=0.008).
8eea62084ca7e541d918e823422bd82e Conclusion
Our study revealed diverse resistance mechanisms to osimertinib in Chinese NSCLC patients and urged for new drug discovery or combination strategies to overcome this clinical challenge.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 5
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-112 - Osimertinib vs Standard of Care (SoC) EGFR-TKI as First-Line Treatment in Chinese Patients With EGFRm Advanced NSCLC (ID 12211)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Osimertinib is an irreversible, central nervous system (CNS) active EGFR-TKI, selective for both EGFRm and T790M resistance mutations. FLAURA (NCT02296125) is a PhIII, double-blind, randomized study assessing efficacy and safety of osimertinib vs SoC EGFR-TKI (erlotinib/gefitinib) in first-line patients with EGFRm advanced NSCLC. FLAURA results (556 patients, globally) are published. We present the China cohort results.
a9ded1e5ce5d75814730bb4caaf49419 Method
The cohort included self-identified Chinese patients, enrolled in China. Eligible patients: ≥18 years, Ex19del/L858R EGFRm advanced NSCLC, no prior EGFR-TKI/systemic anti-cancer therapy for advanced disease. Neurologically stable patients with CNS metastases were allowed, if definitive treatment/corticosteroids were completed ≥2 weeks before enrolment. Patients were randomized 1:1 to osimertinib 80 mg once daily (qd) orally or SoC EGFR-TKI (gefitinib 250 mg qd orally selected by all Chinese sites), stratified by mutation status (Ex19del/L858R). Primary endpoint: progression-free survival (PFS) by RECIST v1.1, per investigator. Data cutoff: 10/01/2018.
4c3880bb027f159e801041b1021e88e8 Result
Overall, 136 patients were randomized (osimertinib n=71; SoC n=65); 19 were also included in the global analysis. Baseline characteristics were balanced across arms (osimertinib/SoC): female 61/71%; smoking history 25/23%; WHO performance status 1 90/80%; Ex19del 51/51%, L858R 49/49%; CNS metastases 24/32%.
Efficacy endpoint Osimertinib
n=71SoC
n=65PFS events, total patients
(% maturity)40
(56%)51
(78%)PFS hazard ratio (HR)*
(95% CI)0.56 (0.37, 0.85); p=0.007 Median PFS, months
(95% CI)17.8
(13.6, 20.7)9.8
(8.3, 13.8)Objective response rate (ORR),
% (95% CI)83%
(72, 91)75%
(63, 85)Median duration of response (DoR), months
(95% CI)16.4
(12.3, NC)10.9
(8.3, 13.8)*A hazard ratio <1 favours osimertinib. PFS benefit was observed across all subgroups, irrespective of EGFR mutation status and including patients with/without CNS metastases at study entry. Median total treatment duration: osimertinib, 18.9 months; SoC, 13.6 months. No new safety signals were reported. Numerical increase in grade ≥3 AEs was reported in the osimertinib arm (49%) versus SoC arm (23%). Most grade ≥3 AEs in the osimertinib arm were investigator-reported laboratory and disease-related AEs; incidence of non-laboratory-related events was low. AEs leading to discontinuation: osimertinib, 13%; SoC, 6%. In the osimertinib arm, most AEs leading to discontinuation were fatal disease-related events.
8eea62084ca7e541d918e823422bd82e Conclusion
Osimertinib improved PFS vs SoC EGFR-TKI (HR: 0.56) as first-line treatment in Chinese patients with EGFRm advanced NSCLC, consistent with the global analysis.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-55 - Unique Genetic Profiles from Cerebrospinal Fluid Could Predict Survival of EGFR-Mutant NSCLC with Leptomeningeal Metastases (ID 12369)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Leptomeningeal metastases (LM) are more frequent in NSCLC with EGFR mutations;and cerebrospinal fluid (CSF) could reveal the unique genetic profiles of LM in our previous studies, but whether they could predict the overall survival (OS) of LM remains unknown.
a9ded1e5ce5d75814730bb4caaf49419 Method
EGFR-mutant NSCLC patients with LM were enrolled,and clinical data and genetic profiles detected by Next-generation sequencing were collected. We further drew nomogram with endpoint of OS after LM, then performed index of concordance (C-index) and survival analysis to evaluate predictive role.
4c3880bb027f159e801041b1021e88e8 Result
In total, 61 patients were enrolled and all with genetic profiles from CSF. Patents with high copy number variations (CNVs) or harboring CDK6, TP53 exon5 or FGF19 in CSF demonstrated significant poorer OS than those without (Fig. 1). Cox regression analysis indicated CNVs, CDK6,CDKN2A,TP53,MET and NTRK1 as prognostic factors and further selected for nomogram (Fig. 2). C-index of nomogram was 0.743, indicating the moderate predictive effect. In the calibration curves, we scored the patients based on the model, using bisection and trisection methods to divide into low and high points groups; and low, medium and high points groups (Fig. 3), and significant difference were found in both the survival analyses (NA versus 7.47months, P<0.01) and (NA, 10.33 versus 4.43 months, P<0.01) respectively. Patients who received Osimertinib after LM seemed to have longer OS than those who did not (14.5 months versus 7.7 months) but without significant difference(P=0.10); however interestingly, in those with EGFR T790M negative who took Osimertinib after LM by themselves obtained survival benefit than those who did not(NA versus 7.7 months, P=0.04), and the results needed to be validated.
8eea62084ca7e541d918e823422bd82e Conclusion
Unique genetic profiles from CSF could well predict OS of LM. High CNVs, CDK6, TP53 exon5 or FGF19 in CSF in CSF may be related to poor prognosis of LM.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-97 - Cluster Trial: Ph2 Biomarker-Integrated Study of Single Agent Alpelisib, Capmatinib, Ceritinib and Binimetinib in advNSCLC (ID 12065)
16:45 - 18:00 | Presenting Author(s): Yi-Long Wu
- Abstract
Background
Several genetically altered signaling pathways have been profiled in NSCLC, enabling advanced management of NSCLC using targeted therapies. This study investigated the therapeutic spectrum of NSCLC with uncommon molecular alterations by allocating patients to treatment arms based on molecular aberrations; targeted therapies alpelisib (PI3Kαi), capmatinib (METi), ceritinib (ALKi), and binimetinib (MEKi) were evaluated.
a9ded1e5ce5d75814730bb4caaf49419 Method
The study was based on the umbrella design. Key objectives: investigate feasibility of using one trial for different agents based on biomarker-integrated analysis, assess anti-tumor activity, characterize safety, tolerability and PK profiles of individual agents. Key eligibility criteria: age ≥18 years; ECOG PS ≤2; failed prior treatment/unsuitable for chemotherapy. Documentation of locally determined molecular alterations before treatment allocation was required (alpelisib, 350 mg QD: PIK3CA mutation/amplification; capmatinib, 400 mg BID (tablet): MET IHC overexpression/amplification; ceritinib, 750 mg QD: ALK or ROS1 rearrangement; binimetinib, 45 mg BID: KRAS, NRAS or BRAF mutation).
4c3880bb027f159e801041b1021e88e8 Result
Sixty-six patients with advNSCLC were enrolled (median age 58 years; 65.2% male: alpelisib, n=2; capmatinib, n=16; ceritinib, n=26; binimetinib, n=22). As of Feb 28, 2018, 10 patients in ceritinib and 2 in binimetinib arms were ongoing. Twenty-four patients had confirmed partial responses (36.4%): alpelisib, 0%; capmatinib, 18.8%; ceritinib, 73.1%; binimetinib, 9.1% (Figure). Longest mPFS (14.4 months) was in ceritinib arm. Among the most common treatment-related AEs: alpelisib: malaise, hyperglycemia, dysgeusia; capmatinib: nausea, anemia, peripheral edema, decreased appetite; ceritinib: diarrhea, vomiting, ALT/AST elevation; binimetinib: mouth ulceration, AST, blood CPK increased, rash. Most AEs were grade 1/2.
8eea62084ca7e541d918e823422bd82e Conclusion
6f8b794f3246b0c1e1780bb4d4d5dc53
Objective responses/tumor shrinkage were observed in the study; highest ORR and mPFS were observed with ceritinib, although patient numbers differed between arms. All treatments were well tolerated; no new safety signals were observed. This study demonstrated the feasibility of an umbrella trial and importance of precision medicine in the management of advNSCLC with uncommon molecular alterations. -
+
P1.01-98 - A Phase IIIb Trial of Afatinib in EGFRm+ NSCLC: Analyses of Outcomes in Patients with Brain Metastases or Dose Reductions (ID 12906)
16:45 - 18:00 | Presenting Author(s): Yi-Long Wu
- Abstract
Background
We previously reported interim results of a large (n=479) open-label, single-arm Phase IIIb study of afatinib in EGFR TKI-naïve patients with EGFRm+ NSCLC, in a setting similar to ‘real-world’ practice (Wu et al, WCLC, 2017). In this broad population of Asian patients, the tolerability profile of afatinib was predictable and manageable. Adverse events (AEs) were consistent with the LUX-Lung 3, 6 and 7 trials; 3.8% of patients discontinued due to drug-related AEs. Progression-free survival (PFS) and time to symptomatic progression (TTSP) was encouraging, in patients with both common and uncommon EGFR mutations. TTSP data suggested effective treatment beyond progression. Here, we assess the impact of baseline brain metastases and use of dose reductions on efficacy outcomes.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients with locally advanced/metastatic EGFRm+ NSCLC were recruited in China, Hong Kong, India, Singapore and Taiwan. Afatinib 40mg/day was given until disease progression (investigator-assessed) or lack of tolerability. Treatment-related AEs could be managed by protocol-specified tolerability-guided dose adjustment.
4c3880bb027f159e801041b1021e88e8 Result
At data cut-off (13 Feb 2017), patient characteristics were as follows: median age, 59.0 years; female, 52.4%; EGFR mutations: Del19+/-L858R+/-uncommon, 86.0%; uncommon only, 14.0%; ECOG PS0, 19.8%; PS1, 78.1%. Prior chemotherapy lines: 0, 59.7%; 1, 30.1%; ≥2, 10.2%.
Overall, dose reductions from 40mg/day to 30mg/day occurred in 119 patients (25%). Incidences of the most frequently reported AEs before and after dose reduction were (any grade): diarrhea, 96/51%; rash/acne, 69/58%; stomatitis, 65/42%; (≥grade 3) diarrhea, 27/4%; rash/acne, 24/11%; stomatitis, 11/5%. A total of 96 patients had a dose reduction during the first six months; median PFS in this subgroup was 14.1 months (95% CI: 10.0–19.3) versus 11.33 (10.7–13.6) months in those who remained on the starting dose (n=383); HR=1.37 (1.01–1.85), p=0.041. Median TTSP was 17.7 (13.5–23.7) and 14.7 (12.7–17.0) months, respectively; HR=1.26 (0.92–1.72), p=0.15.
Among 92 patients (19.2%) with brain metastases at baseline, median PFS was 10.9 (8.3–14.3) months, versus 12.4 (10.8–13.9) months in those without metastases (n=387); HR=1.23 (0.91–1.65), p=0.18. Median TTSP was 14.8 (12.7–20.7) and 15.4 (12.9–18.0) months, respectively; HR=1.0 (0.71–1.40), p=1.0.
8eea62084ca7e541d918e823422bd82e Conclusion
These findings demonstrate that tolerability-guided dose adjustment of afatinib is an effective measure to reduce treatment-related AEs, while maintaining therapeutic efficacy. TTSP was similar between patients with and without brain metastasis. This is additional evidence for the efficacy of afatinib in patients with brain metastases.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-99 - Detecting HER2 Alterations by Next Generation Sequencing (NGS) in Patients with Advanced NSCLC from the United States and China (ID 11285)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Advances in NGS have led to an increase in identifying specific actionable gene alterations across tumor types. We collected data on HER2 gene alterations detected by NGS from patients with advanced NSCLC and analyzed clinical characteristics and HER2 targeted treatments.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients diagnosed with advanced NSCLC and underwent NGS testing from Jun 2014 to Dec 2017 at Memorial Sloan-Kettering Cancer Center (MSK) and Guangdong General Hospital (GGH) were included. NGS platforms were MSK-IMPACTTM in MSK and GeneSeek or BurnStone in GGH. Descriptive statistics are used in data analysis.
4c3880bb027f159e801041b1021e88e8 Result
2200 patients from MSK and 490 patients from GGH underwent NGS testing. HER2 mutation and/or amplification were detected in 91/2200(4.1%) patients and 28/490(5.7%) patients from MSK and GGH respectively. Clinical characteristics were listed in Table1. 37.4%(34/91) and 21.4%(6/28) patients from MSK and GGH received HER2 targeted therapies. More patients were enrolled to HER2 inhibitors clinical trials in MSK(24.2%) than GGH(7.1%). The characteristics of HER2 alterations are summarized in Table2.
Table 1. Comparison of HER2 alterations in advanced NSCLC patients from U.S. and China
MSK
N (%)
GGH
N (%)
Total Patients
91
28
Age at Diagnosis (years)
<=60
34 (37.4%)
13 (46.4%)
>60
57 (62.6%)
15 (53.6%)
Sex
Male
37 (40.7%)
14 (50%)
Female
54 (59.3%)
14 (50%)
Smoking History
Former/Current Smoker
53 (58.2%)
7 (25%)
Non-Smoker
38 (41.8%)
21 (75%)
Histology
Adenocarcinoma
84 (92.3%)
25 (89.3%)
Squamous Cell Carcinoma
5 (5.5%)
0
Misc
2 (2.2%)
3 (10.7%)
HER2 status
Mutation
48 (52.7%)
16 (57.1%)
Amplification
32 (35.2%)
11 (39.3%)
Mutation + Amplification
11 (12.1%)
1 (3.6%)
HER2 targeted treatment
34 (37.4%)
6 (21.4%)
Enrolled to HER2 inhibitors clinical trials
22 (24.2%)
2 (7.1%)
8eea62084ca7e541d918e823422bd82e ConclusionTable 2. HER2 alteration in advanced NSCLC patients from U.S. and China combined
NGS Result
Mutation Only
N (%)
Amplification Only
N (%)
Mutation + Amplification
N (%)
Total Patients 64 43 12 Age at Diagnosis (years)
<=60
31 (48.4%)
20 (46.5%)
8 (66.7%)
>60
33 (51.6%)
23 (35.9%)
4 (33.3%)
Sex
Male
39 (60.9%)
19 (44.2%)
7 (58.3%)
Female
25 (39.1%)
24 (55.8%)
5 (41.7%)
Smoking History
Former/Current Smoker
31 (48.4%)
24 (55.8%)
5 (41.7%)
Non-Smoker
33 (51.6%)
19 (44.2%)
7 (58.3%)
Histology
Adenocarcinoma
58 (90.6%)
39 (90.7%)
12 (100%)
Squamous Cell Carcinoma
1 (1.6%)
4 (9.3%)
0
Misc
5 (7.5%)
0
0
HER2 targeted treatment
Yes
19 (29.7%)
14 (32.6%)
7 (58.3%)
No
45 (70.3%)
29 (67.4%)
5 (41.7%)
The incidence and clinical characteristics of HER2 alterations in advanced NSCLC were similar between two large cancer centers in the U.S. and China. These data support U.S.-China collaboration in clinical trials for patients with rare molecular subsets of NSCLC to accelerate new cancer drug development.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.03 - Biology (Not CME Accredited Session) (ID 935)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.03-34 - Combined Molecular and Radiological Evaluation Unveils Three Subtypes of Disease Progression to a Third Generation EGFR TKI (ID 12055)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
The definition of disease progression (PD) to EGFR TKIs has evolved from RECIST to a combination of clinical and RECIST evaluation. Patients with dramatic, local or gradual progression to third generation EGFR TKIs have been tailored to different subsequent treatment strategies. However, little is known about progression to third generation EGFR TKIs from molecular perspective.
a9ded1e5ce5d75814730bb4caaf49419 Method
Longitudinal plasma samples were collected from T790M-positive patients who progressed on a third generation EGFR TKI AC0010 in a phase I/II study in Guangdong Lung Cancer Institute. A pre-defined and unified molecular and radiological evaluation of PD were performed. Ultra-deep sequencing capturing 295 cancer-related genes was performed to track the changes in ctDNA to depict molecular PD, which was defined by acquired SNV/SCNV, or ≥20% increase in allelic fraction/copy number of pre-existing SNV /SCNV or both. Radiological PD was defined by RECIST.
4c3880bb027f159e801041b1021e88e8 Result
As of October 2016, 102 serial plasma samples from 23 patients with clinical PD were included. Three subtypes of PD to AC0010 were revealed (Fig1). Molecular PD occurred prior to radiological PD in 43.5% of patients (10/23), with an average lead time of 3.0 months. Molecular PD occurred concurrently with radiological PD in 39.1% of patients (9/23). Interestingly, 17.4% of patients (4/23) experienced radiological PD prior to molecular PD, with molecular PD occurred during AC0010 continuation beyond progression (CBPD) in 3 patients. Of patients experienced clinical stable PD in extracranial lesions, radiological PD occurring prior to molecular PD group (n=2) demonstrated longer duration of AC0010 CBPD than molecular PD occurring prior to (n=3) or concurrently with radiological PD groups (n=4) (Median, 5.6 months vs. 1.9 months vs. 1.8 months).
8eea62084ca7e541d918e823422bd82e Conclusion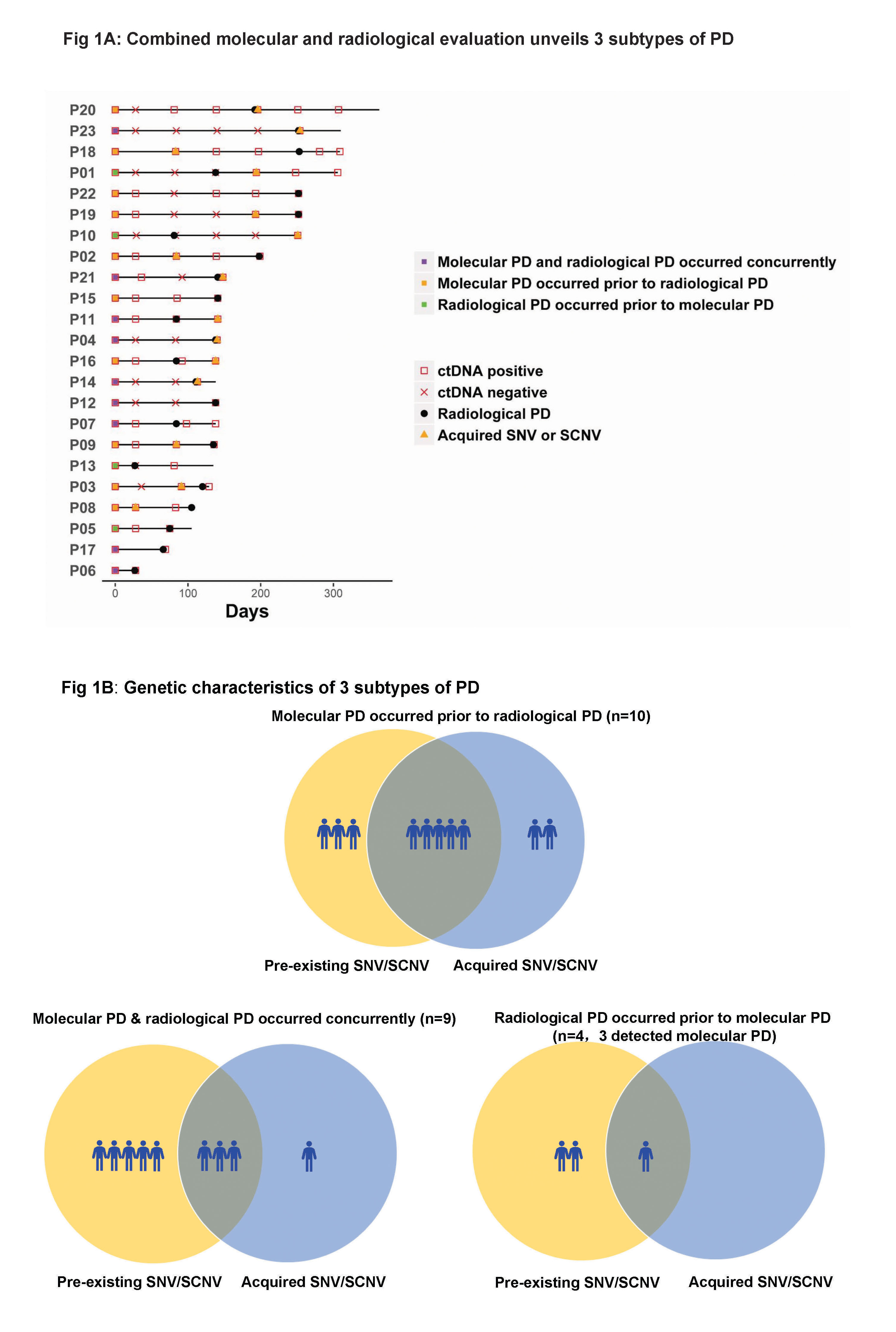
Our study revealed 3 distinct subtypes of PD to AC0010, providing insights into PD by combining molecular and radiological evaluation and might guide the optimal time for treatment switch and personalized subsequent treatments.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.11 - Screening and Early Detection (Not CME Accredited Session) (ID 943)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.11-18 - A Classification-Based Machine Learning Method Reveals Exosomal miRNA Biomarkers for Patients with Pulmonary Ground Glass Nodule (ID 12462)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Non-invasive detection of lung cancer is of critical importance but has proven challenging due to the rate of false-negative diagnosis with current tests. Plasma exosomes have been implicated as a non-invasive diagnostic source. However, little high throughput screening has been done in the early-stage lung cancer and problems such as bias of enrollment, less rigorous identification exists. This study aimed to reveal the plasma exosome-derived miRNA biomarkers for early-stage lung cancer patients, especially those with ground glass nodule (GGN).
a9ded1e5ce5d75814730bb4caaf49419 Method
Pre-operative and paired post-operative plasma samples from patients with solitary pulmonary nodule and healthy volunteers were prospectively collected. Finally 38 malignant nodules, 7 benign nodules and 5 healthy volunteers were enrolled. The malignant nodules included 9 pure GGNs, 11 mixed GGNs and 18 solid nodules. Exosomes were collected from 1mL plasma and were isolated with 3D Medicine EV isolation kit. Exosomal miRNA profiling was performed using miRNA-seq. And an exosomal miRNA diagnostic model for patients with malignant nodules was constructed by using support vector machine (SVM).
4c3880bb027f159e801041b1021e88e8 Result
In general, malignant nodules, benign nodules and healthy volunteers were indistinguishable based on overall clustering. Regarding to malignant nodules, pure GGNs and solid nodules could be separated under principal component analysis (PCA), and the mixed GGNs presented a transitional state between the pure GGNs and the solid nodules. Ultimately, a two-dimensional SVM diagnostic model for discriminating malignant and benign nodules was established. The optimal miRNA combination could reach an area under curve (AUC) of 0.96, with sensitivity and specificity of 94.7% and 91.7%, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
This preliminary analysis highlights the potential of exosomal miRNA based liquid biopsy for non-invasive detection of early-stage lung cancer. The SVM model seems could effectively distinguish pulmonary nodules, but needs further verified.
6f8b794f3246b0c1e1780bb4d4d5dc53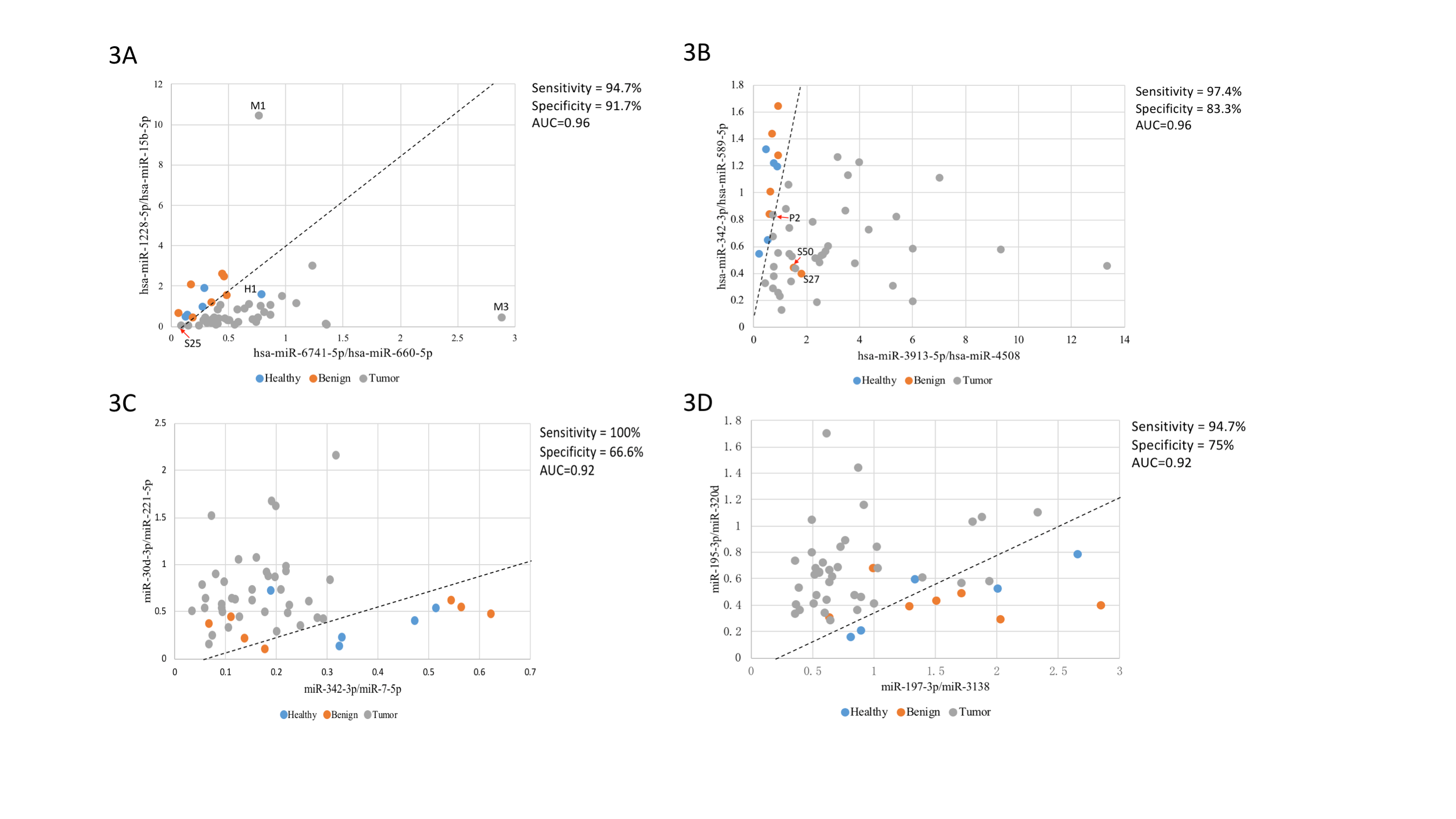
-
+
P1.13 - Targeted Therapy (Not CME Accredited Session) (ID 945)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.13-02 - eXalt3: Phase 3 Randomized Study Comparing Ensartinib to Crizotinib in Anaplastic Lymphoma Kinase Positive Non-Small Cell Lung Cancer Patients (ID 13294)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Ensartinib (X-396) is a novel, potent ALK small molecule tyrosine kinase inhibitor (TKI). It is well-tolerated and has shown promising activity in NSCLC patients in a phase 1/2 study in patients that were both ALK TKI naïve and patients that received prior crizotinib, as well as those with CNS metastases. The safety profile of ensartinib appears to be different from other ALK TKIs.
a9ded1e5ce5d75814730bb4caaf49419 Method
In this global, phase 3, open-label, randomized study, approximately 270 patients with ALK+ NSCLC who have received no prior ALK TKI and up to one prior chemotherapy regimen will be randomized with stratification by prior chemotherapy (0/1), performance status (0-1/2), brain metastases at screening (absence/presence), and geographic region (Asia /other), to receive oral ensartinib (225 mg, once daily) or crizotinib (250mg, twice daily) until disease progression or intolerable toxicity.
Eligibility also includes patients ≥ 18 years of age, stage IIIB or IV ALK+ NSCLC. Patients are required to have measurable disease per RECIST 1.1, adequate organ function, and an ECOG PS of ≤2. Adequate tumor tissue (archival or fresh biopsy) must be available for central testing. The primary endpoint is progression-free survival assessed by independent radiology review based on RECIST v. 1.1 criteria. Secondary efficacy endpoints include overall survival, response rates (overall and central nervous system [CNS]), PFS by investigator assessment, time to response, duration of response, and time to CNS progression. The study has > 80% power to detect a superior effect of ensartinib over crizotinib in PFS at a 2-sided alpha level of 0.05.
Phase 3 recruitment began in June, 2016 and currently has 98 active sites in 21 countries. The duration of recruitment will be approximately 28 months. This study is registered with ClinicalTrials.Gov as NCT02767804.
4c3880bb027f159e801041b1021e88e8 Result
Section not applicable
8eea62084ca7e541d918e823422bd82e Conclusion
Section not applicable
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-18 - Differential Molecular Mechanisms Associated with Dramatic and Gradual Progression in NSCLC Patients with Intrathoracic Dissemination (ID 12979)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Lung cancer is a highly heterogeneous disease with diverse clinical outcomes. The pleural cavity is a frequent metastasis site of proximal lung cancer. Better understanding of its underlining molecular mechanisms associated with dramatic and gradual progression of pleural metastasis in patients with non-small cell lung cancer (NSCLC) is essential for prognosis, intervention and new therapy development.
a9ded1e5ce5d75814730bb4caaf49419 Method
We performed whole-exome sequencing (WES) of matched primary lung adenocarcinoma and pleural metastatic tumors from 26 lung cancer patients with dramatic progression (DP, n=13) or gradual progression (GP, n=13). Somatic alterations at both genome-wide level and gene level were detected. Kaplan-Meier survival analysis and multivariate Cox regression models were applied to analyze the association between different somatic alterations and clinical parameters.
4c3880bb027f159e801041b1021e88e8 Result
We first analyzed the differences in somatic alterations between AP and RP group in the primary tumors, and identified higher somatic copy number alteration (SCNA) level in DP group compared to GP group, which is significantly (p=0.016) associated with poorer progression-free survival (PFS). More specifically, patients with chromosome 18q loss in the primary tumor showed a trend (p=0.107) towards poorer PFS. PTEN (p=0.002) and GNAS (p=0.002) mutations are enriched in the primary tumors of DP group, and are associated with poorer PFS. Furthermore, pleural metastatic tumors harbor a relatively higher level of mutation burden (p=0.105) and significantly increased SCNA (p=0.035) compared to the primary tumors.
8eea62084ca7e541d918e823422bd82e Conclusion
NSCLC patients in the attenuated progression group have more stable genomes. High level of genomic instability, GNAS and PTEN mutations, as well as chromosome 18q loss are associated with rapid progression.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01-52 - Identification of Leptomeningeal Metastasis-Specific Exosomal miRNA Signatures in Cerebrospinal Fluids of NSCLC Patients (ID 13074)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Leptomeningeal metastasis (LM) is a devastating complication with poor prognosis in non-small-cell lung cancer (NSCLC) patients. The confirmed diagnosis of LM usually involves neurological evaluation, MRI imaging, and cytopathology analysis of limited tumor cells from cerebrospinal fluid (CSF). Exosomes are extracellular vesicles in body fluids enriched with microRNAs (miRNAs), which have been implicated to participate in brain metastasis. Here, we aimed to identify LM-specific exosomal miRNA signatures in NSCLC patients to elucidate their potential role in LM mechanism and to predict LM via liquid biopsy.
a9ded1e5ce5d75814730bb4caaf49419 Method
Exosomes prepared from CSF and plasma samples of 39 advanced NSCLC patients with (LM+) or without (LM-) LM as well as 12 non-cancer individuals (NC) were underwent small RNA next-generation sequencing. For patients in the LM+ group, paired plasma samples were taken before (PLM+pre) and upon (PLM+post) LM diagnosis. Exosomal miRNA profiles were subjected for differential expression analysis, pathway enrichment analysis, and signature discovery.
4c3880bb027f159e801041b1021e88e8 Result
Unsupervised hierarchical clustering of the miRNA expression profiles clearly separated CSF samples into LM+ and LM free groups (LM- and NC). Interestingly, these samples were stratified based on their LM status only, regardless of their intraparenchymal metastasis status. In total, 247 (185 up and 62 down-regulated) miRNAs were identified differentially presented in the LM+ CSF exosome samples compared to the LM- and NC groups. Top altered miRNAs include dramatically up-regulated miR-200 family and down-regulated miR-144/451 cluster. Predicted gene targets of these top-regulated miRNAs were significantly enriched in Ras/MAPK/PI3K-AKT signaling, endocytosis pathways, and so on. Promisingly, a signature of five CSF exosomal miRNAs (let-7e-5p, miR-28-3p, miR-375, miR-200a-3p, and miR-486-5p) was identified for classification of LM+ patients with 100% sensitivity and 100% specificity. Due to the higher background complexity, we only identified one miRNA (miR-24-3p) was significantly up-regulated and one miRNA (miR-92b-5p) was significantly down-regulated in LM+ patients’ plasma-derived exosomes (PLM+pre and PLM+post) compared with the LM free group (PLM- and PNC). However, a combined signature of seven miRNAs (miR-24-3p, miR-223-3p, miR-340-5p, miR-27a-3p, miR-423-5p, miR-2110 and miR-342-5p) from PLM+pre samples was identified for the prediction of future LM with 81% sensitivity and 76% specificity.
8eea62084ca7e541d918e823422bd82e Conclusion
NSCLC patients with LM present a remarkably distinct CSF exosomal miRNA signature, which may involve in the progression of LM, and can be used as diagnostic biomarkers for LM. Furthermore, the identification miRNA signature in the pre-LM plasma samples suggests the potential use of liquid biopsy to predict LM for better patient care.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.04 - Immunooncology (Not CME Accredited Session) (ID 953)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.04-29 - Preliminary Results With Tislelizumab in Chinese Patients With Non-Small Cell Lung Cancer (NSCLC) (ID 11319)
16:45 - 18:00 | Presenting Author(s): Yi-Long Wu
- Abstract
Background
NSCLC accounts for 80–85% of all lung cancers and has a poor prognosis at later stages. Immune checkpoint inhibitors have shown efficacy in patients (pts) with advanced NSCLC. Tislelizumab is a humanized IgG4 monoclonal antibody with high affinity/specificity for PD-1. Tislelizumab was specifically engineered to minimize FcϒR binding on macrophages that, based on preclinical evidence, is believed to minimize potentially negative interactions with other immune cells. In a phase 1 study, tislelizumab was generally well tolerated and showed antitumor activity in NSCLC pts; 200 mg IV Q3W was established as the recommended tislelizumab dose.
a9ded1e5ce5d75814730bb4caaf49419 Method
In the ongoing indication-expansion phase of this study, Chinese pts with histologically confirmed NSCLC were enrolled into PD-L1-high (PD-L1+; ≥10% tumor cells expressing PD-L1) and PD‑L1‑low (PD-L1–) cohorts. Antitumor activity (RECIST v1.1) and safety/tolerability (NCI-CTCAE v4.03) were assessed.
4c3880bb027f159e801041b1021e88e8 Result
As of 8 Dec 2017, 42 NSCLC pts (median age 54 yr [range 37–72]) were enrolled; 17 were PD-L1+ and 25 were PD-L1–. Most pts were male (69%), former/current smokers (57%), and had received prior therapy (95%). Adenocarcinoma was the most prevalent histology (57%). Median follow-up was 4.5 mo and 23 pts remain on treatment. Of the 39 response-evaluable pts, 4 (n=2/14, PD-L1+; n=2/25, PD-L1–) achieved confirmed PR and 20 (n=6/14, PD-L1+; n=14/25, PD‑L1–) achieved SD, including 4 (n=2, PD-L1+; n=2, PD‑L1–) with unconfirmed PR. Across the study population, ORR was 10% and DCR was 61.5%. ORRs by cohort were 14% (PD‑L1+) and 8% (PD-L1–), respectively. Common treatment-related AEs were increased AST (24%), increased ALT (19%), hypothyroidism (12%), and rash (12%). Five grade ≥3 treatment-related AEs occurred in 4 pts (increased AST [n=2], hyperglycemia, increased ALT, and increased GGT [n=1 each]). No treatment-related grade 5 events were reported.
8eea62084ca7e541d918e823422bd82e Conclusion
Tislelizumab was generally well tolerated and demonstrated antitumor activity in previously treated pts with advanced NSCLC. A global phase 3 study (NCT03358875) of tislelizumab vs docetaxel as potential second/third-line therapy in NSCLC pts who progressed after a platinum-based regimen is ongoing.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.04-30 - PD-1/PD-L1 Inhibition Might be an Option for the Treatment of Advanced Primary Pulmonary Lymphoepithelioma-Like Carcinoma (ID 12698)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a rare subtype of non-small-cell lung cancer mostly reported in Asian countries, which is frequently associated with Epstein-Barr virus (EBV) infection. There is no consensus on the choice for the treatment of advanced primary pulmonary LELC. The utility of PD-1/PD-L1 inhibitor to this cancer type remains poorly understood.
a9ded1e5ce5d75814730bb4caaf49419 Method
From January 2008 to April 2017, a total of 53 patients receiving surgical removal and diagnosed as primary pulmonary LELC in Guangdong Lung Cancer Institute (GLCI) were enrolled in this study. Sections formalin-fixed and paraffin-embedded (FFPE) tumor samples were stained with PD-L1 antibody (clone E1L3N, Cell Signaling Technology) by immunohistochemistry (IHC). PD-L1 expression more than 1% in tumor cells was considered as PD-L1 positive. Moreover, we reviewed the medical records of 13 primary pulmonary LELC patients who received the treatment of PD-1/PD-L1 inhibitors in GLCI. Their clinicopathological characteristics and relevant prognostic data were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
Among the 53 patients with operable disease, the median age was 56 (36-78), there were 26 males and 27 females, 15 smokers and 38 nonsmokers. Positive rates of PD-L1 in the early pulmonary LELC were 78.8% (41/52,one specimen can't evaluate) and 73.1% (38/52) and 23.1% (12/52) respectively at the cutoff values of 1% and 5% and 50% positivity in tumor cells. ORR of PD-1/PD-L1 inhibition in the evaluable 12 patients with advanced LELC was 16.7% (2/12), and DCR was 75.0% (9/12). In the 6 patients with positive PD-L1 expression, ORR was 33.3% (2/6), DCR was 100.0% (6/6). The two responder patients got 55% and 64% shrinkage of the tumors respectively. All patients had no EGFR mutations.

8eea62084ca7e541d918e823422bd82e Conclusion
This preliminary study showed that pulmonary LELCs have remarkably high incidence of PD-L1 expression. PD-1/PD-L1 inhibitors might be an option for the treatment of advanced primary pulmonary LELC, which needs further investigation.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 961)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.12-11 - A Prognostic Model Integrating Immunohistochemistry Markers for Extensive-Disease Small-Cell Lung Cancer (ID 11828)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Extensive-disease small-cell lung cancer (ED-SCLC) is a subtype of high-grade neuroendocrine carcinoma (HGNEC) with poor prognosis. We tend to build a prognostic nomogram and illustrate the failure pattern of first line etoposide/Irinotecan with paclitaxel (EP/IP) treatment.
a9ded1e5ce5d75814730bb4caaf49419 Method
250 ED-SCLC patients received first line EP/IP treatment were enrolled. Cox regression analysis was used to identify the prognostic factors to establish nomogram. The predictive accuracy of nomogram was evaluated by concordance index (C-index). Further stratification based on Ki67 and brain metastasis was performed through X-tile plot and Kaplan Meier.
4c3880bb027f159e801041b1021e88e8 Result
Cox regression analysis indicated brain metastasis as the prognostic factor and we further selected NSE, gender, TTF-1, Syn, tumor size and smoking status under clinical consideration for nomogram. C-index of nomogram suggested 0.65 with moderate predictive effect. Subgroup analysis showed patients with Ki67 lower than 85% had poorer prognosis than those over 90% (HR 0.59, 95%CI 0.39-0.92, p=0.02). Those without brain metastasis at baseline achieving partial response (PR)/complete response (CR) suggested no prognostic significance in brain progression compared to other progression group.
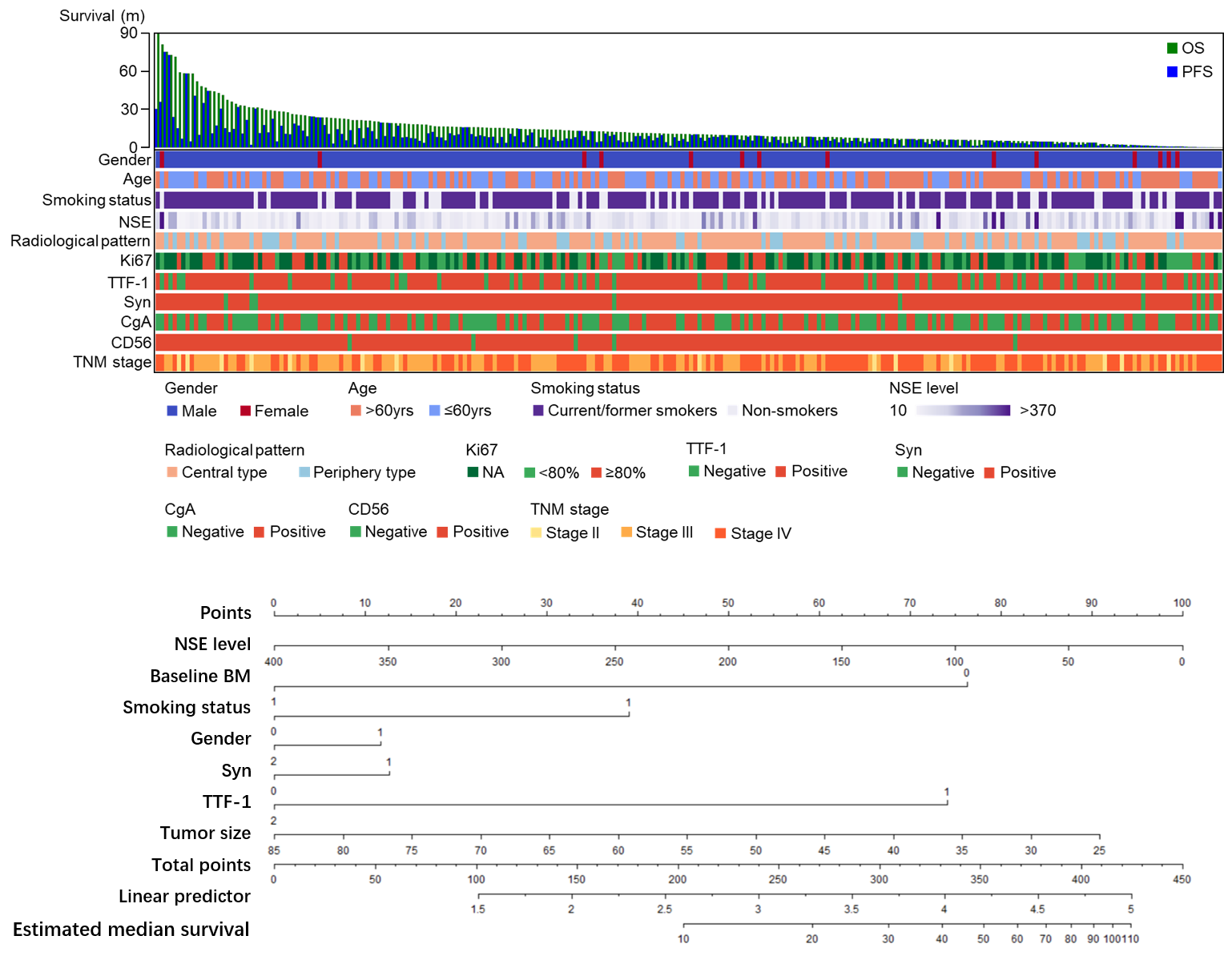
8eea62084ca7e541d918e823422bd82e Conclusion
Established nomogram could well predict prognosis in ED-SCLC. Ki67 might play a potential role in prognosis of SCLC. Application of preventive cranial irradiation might be challenged in ED-SCLC patients without brain metastasis.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.17 - Treatment of Locoregional Disease - NSCLC (Not CME Accredited Session) (ID 966)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.17-32 - Dynamic Monitoring Before and After Neo-Adjuvant Crizotinib in Non-Small Cell Lung Cancer: A Brief Report (ID 11829)
16:45 - 18:00 | Author(s): Yi-Long Wu
- Abstract
Background
Neo-adjuvant therapy has been considered as an optional approach for locally advanced non-small-cell lung cancer (NSCLC) patients. While targeted therapy has been widely applied in advanced NSCLC, neo-adjuvant targeted therapy remains poorly explored.
a9ded1e5ce5d75814730bb4caaf49419 Method
We describe four ALK-positive patients with pathological confirmed locally advanced NSCLC receiving neo-adjuvant Crizotinib. All patients received Crizotinib at a starting dose of 250mg twice daily for 1-3 months before surgical resection. One patients provided dynamic monitoring before and after neo-adjuvant therapy through next generation sequencing of plasma and tissue.
4c3880bb027f159e801041b1021e88e8 Result
Three patients were partial response without apparent adverse event before surgery while one received pathological complete response to neo-adjuvant Crizotinib but suffering from grade 4 hepatic damage. One of them had disease recurrence but achieved long duration of response (PFS=15m) through first-line Crizotinib. Dynamic monitoring with both plasma and tissue indicated simultaneously decrease of sensitive ALK-signaling in a patient with partial response (-51%) and no ALK-dependent resistant variants were captured.


8eea62084ca7e541d918e823422bd82e Conclusion
Neo-adjuvant Crizotinib may be feasible and well-tolerated in locally advanced disease for complete resection. Crizotinib prior to surgery may provide thorough elimination of circulating molecular residual disease and it did not influence the response of reusing Crizotinib in first-line setting.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.01 - Advanced NSCLC (Not CME Accredited Session) (ID 967)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.01-113 - A Multicenter Survey of One Year Survival Among Chinese Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer (CTONG1506) (ID 12337)
12:00 - 13:30 | Author(s): Yi-Long Wu
- Abstract
Background
Previous results of CTONG1506 study showed that gene aberration test rate was increasing in Chinese NSCLC patients and first-line treatment was standardized accordingly. This survey further described one year survival of patients with different gene aberration status and under different first-line treatments.
a9ded1e5ce5d75814730bb4caaf49419 Method
CTONG1506 was a two-year series cross-sectional study. Patients with advanced nonsquamous NSCLC who were admitted from August 2015 to March 2016 and who received first-line anti-cancer treatment at one of 12 tertiary hospitals across China were included. Data extracted from medical charts were entered into medical record abstraction forms, which were collated for analysis. Survival information was collected one year after patients were admitted to hospital. One year survival rate and its 95% confidence interval were analysed by Kaplan-Meier method.
4c3880bb027f159e801041b1021e88e8 Result
A total of 707 patients were analysed, with mean age of 57 years and 56.7% were male. Among the 487 patients who had survival data, 192 were EGFR- mutation positive (86 mutated in exon 19 [one year survival rate 0.90, 95% CI: 0.81-0.94] and 88 mutated in exon 21 [one year survival rate 0.84, 95% CI: 0.75-0.90]), 27 patients were ALK positive and 164 patients were EGFR and ALK wild type. Most EGFR mutation positive patients (128/192) received tyrosine kinase inhibitors (TKIs) as first-line treatment and most EGFR wild type patients (155/175) received first-line chemotherapy (Chemo). Pemetrexed was the most common non-platinum chemotherapy-backbone agent (120/155) in platinum doublet regimens. One year survival rates are shown in the table.
8eea62084ca7e541d918e823422bd82e Conclusion
This national-wide real world study of tertiary hospitals in China revealed that a majority of (>75%) advanced nonsquamous NSCLC patients survived more than one year and was comparable to well-controlled clinical trial results, indicating survival benefits by gene aberration status guided standard of care. This result may be further validated by our on-going two-year survey.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P3.01-64 - Preliminary Data of Diverse Therapies in Patients with Advanced Non–Small-Cell Lung Cancer Harbouring RET-Rearrangement (ID 13677)
12:00 - 13:30 | Author(s): Yi-Long Wu
- Abstract
Background
Activating RET-rearrangement has been discovered to play a crucial role in NSCLC tumorigenesis. However, the lack of specificity narrowed efficacy of multi-kinase inhibitors (MKIs) and the optimal treatment remains unknown. In this study, we compared chemotherapy, immunotherapy and MKIs in this group of patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively evaluated the efficacy of these three treatments in advanced, RET-rearranged NSCLC patients between January 2013 and April 2018 at our institution. RET-rearrangements were assessed by Next-generation sequencing (NGS) or any of FISH, IHC, RT-PCR. Treatment data were collected after the patients had been diagnosed with RET-rearranged advanced NSCLC. Progression-free survival (PFS) was measured from treatment start to disease progression, all-cause mortality or last follow up. Median follow-up time was 5.1months. NGS was performed to assess somatic mutation of available samples.
4c3880bb027f159e801041b1021e88e8 Result
A total of 30 patients with RET-rearrangement were investigated in this study. After the diagnosis, 15 patients, genetic profiles confirmed by NGS, received chemotherapy (n=10), checkpoint-inhibitors (n=7) and RET targeted MKI (n=6) with evaluable response. Several patients take any two of these three treatments as different line therapies. The disease control rate of chemotherapy, immunotherapy, MKI group was 70.0%, 71.43% and 50%, respectively. While the median PFS of three groups was 2.50 months, 2.70 months, 0.30 months, respectively, which of no significance. The NGS data of 10 patients showed that RET-rearrangement co-occurred with several other genes, including TP53, NTRK, CDK4, ERBB4. A low mutation burden (mean 4.5 mutations) was observed (Figure 1).
8eea62084ca7e541d918e823422bd82e Conclusion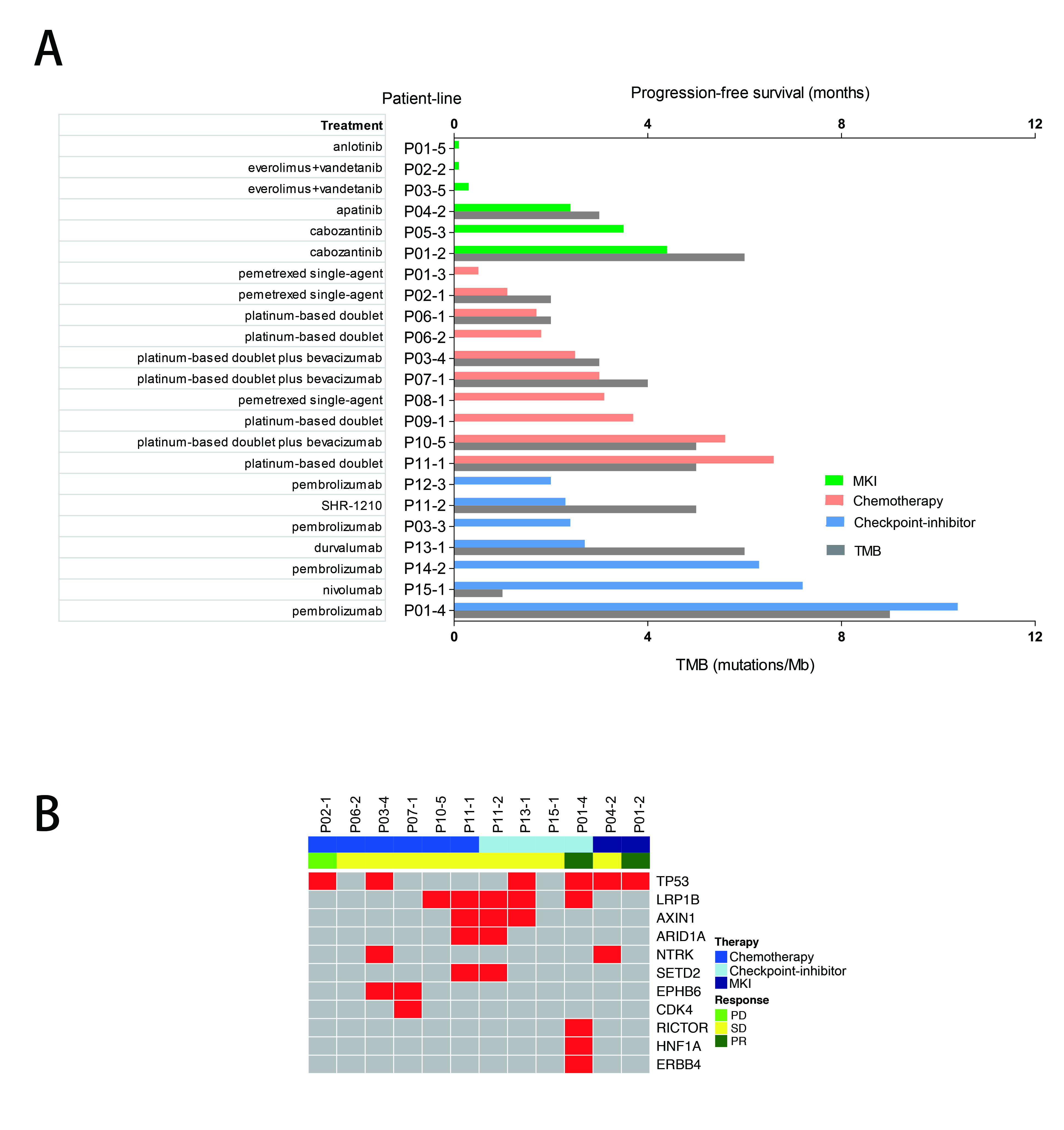
We confirmed relatively low PFS in advanced RET-rearranged NSCLC with MKIs reported in previous studies. But further investigation is warranted. Treatment with checkpoint-inhibitors seemed to encouragingly prolong PFS but a larger group of patients is needed to draw a definite conclusion.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 978)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.12-10 - Immunogenomic Characteristics of SCLC and LCNEC Redefined Molecular Subgroups (ID 12577)
12:00 - 13:30 | Author(s): Yi-Long Wu
- Abstract
Background
While small-cell lung cancer (SCLC) and large-cell neuroendocrine carcinoma (LCNEC) are distinct classes of high-grade neuroendocrine carcinomas, the differential diagnosis between SCLC and LCNEC remains challenging. In fact SCLC and LCNEC overlap in clinical, histopathologic, cytologic, morphologic and genetic characteristics. Molecular profiling with microarray or next-generation sequencing has provided growing evidence suggesting that both SCLC and LCNEC are biologically heterogeneous and a great part of them are borderline neuroendocrine carcinomas falling between typical SCLC and LCNEC. On account of accumulated knowledge, we speculated that immunogenomically characterizing SCLC and LCNEC collectively as one group, or rather morphologically or cytologically separating SCLC from LCNEC has superior clinical value.
a9ded1e5ce5d75814730bb4caaf49419 Method
We analyzed gene expression profiles of 44 SCLCs, 56 LCNECs and 25 normal lung samples obtained from Gene Expression Omnibus. Unsupervised and supervised analyses were performed to understand molecular characteristics of samples. Pathway and CIBERSORT analyses were employed to obtain immune landscape of SCLC and LCNEC.
4c3880bb027f159e801041b1021e88e8 Result
Unsupervised clustering with 1189 differentially expressed genes revealed 2 distinct molecular subgroups (G1 and G2) of SCLC and LCNEC, which is not associated with histopathology. Targeted pathway analysis found that G1 was marked by activated IL-17, MAPK and Hippo signaling pathways. In contrast, transcriptional factors, such as ASCL1, INSM1, SOX2, and NKX2-1 were significantly up-regulated in G2, but not in G1. Moreover, in silico analysis of cellular composition and expression of immune genes disclosed unique immunoprofiles for G1 and G2. G1 was characterized by enriched CD4 memory cells, M1 macrophages and activated dendritic cells. While G2 was composed of high fractions of memory B cells and naïve CD4 cells. Strikingly, expression of both immunoinhibitors (IL10, PDL1, IDO1) and immunomodulators (OX40L, BAFF, GITR, IL6), as well as MHC class I and II molecules was higher in G1 compared to that in G2.
8eea62084ca7e541d918e823422bd82e Conclusion
We identified the common intrinsic features and molecular subgroups of SCLC and LCNEC, which are beyond conventional histopathology and better associated with immunogenomics of tumors. Further research is warranted to identify potential clinical implication of SCLC and LCNEC molecular subgroups.
6f8b794f3246b0c1e1780bb4d4d5dc53


