Virtual Library
Start Your Search
Baohui Han
Author of
-
+
JCSE01 - Perspectives for Lung Cancer Early Detection (ID 779)
- Event: WCLC 2018
- Type: Joint IASLC/CSCO/CAALC Session
- Track: Screening and Early Detection
- Presentations: 2
- Moderators:
- Coordinates: 9/23/2018, 07:30 - 11:15, Room 202 BD
-
+
JCSE01.19 - ALTER-0303 Study: Tumor Mutation Index (TMI) For Clinical Response to Anlotinib in Advanced NSCLC Patients at 3rd Line (ID 14708)
11:15 - 11:15 | Presenting Author(s): Baohui Han
- Abstract
Background
Anlotinib is an effective multi-targeted receptor tyrosin kinase inhibitor (TKI) for refractory advanced Non-Small Cell Lung Cancer (NSCLC) therapy at 3rd line. ALTER-0303 clinical trial has been revealed that Anlotinib significantly prolongs progression free survival (PFS; Anlotinib: 5.37 months vs Placebo: 1.40 months) and overall survival (OS; Anlotinib: 9.63 months vs Placebo: 6.30 months) with the objective response rate (ORR) of 9.18% and the disease control rate (DCR) of 80.95%. Here, we sought to understand the gene mutation determinants for clinical response to Anlotinib via next generation sequencing (NGS) upon cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA) at baseline.
Totally 437 advanced NSCLC patients enrolled in ALTER-0303 study, and 294 patients received Anlotinib therapy. Of the 294 patients, 80 patients were analyzed in the present study. Capture-based targeted ultradeep sequencing was performed to obtain germline and somatic mutations in cfDNA and ctDNA. Response analyses upon discovery cohort (n = 62) and validation cohort (n = 80) were performed by use of germline and somatic (G+S) mutation burden, somatic mutation burden, nonsynonymous mutation burden, and unfavorable mutation score (UMS), respectively. Based on the above independent biomarkers and their subtype factors, tumor mutation index (TMI) was developed, and then used for response analysis.
Our data indicated that the patients harbouring less mutations are better response to Anlotinib therapy (G+S muatation burden, cutoff = 4000, Median PFS: 210 days vs 127 days, p = 0.0056; somatic mutation burden, cutoff = 800, Median PFS: 210 days vs 130 days; p = 0.0052; nonsynonymous mutation burden, cutoff = 50, Median PFS: 209 days vs 130 days; p = 0.0155; UMS, cutoff = 1, Median PFS: 210 days vs 131 days; p = 0.0016). TMI is an effective biomarker for Anlotinib responsive stratification (Median PFS: 210 days vs 126 days; p= 0.0008; AUC = 0.76, 95% CI: 0.62 to 0.89) upon discovery cohort and validation cohort (Median PFS: 210 days vs 127 days; p = 0.0006). Lastly, integrative analysis of TMI and IDH1 mutation suggested a more promising result for Anlotinib responsive stratification upon validation cohort (Median PFS: 244 days vs 87 days; p < 0.0001; AUC = 0.90, 95% CI: 0.82 to 0.97).This study provide a biomarker of TMI to stratify Anlotinib underlying responders, that may improve clinical outcome for Anlotinib therapy on refractory advanced NSCLC patients at 3rd line. Clinical trial information: NCT02388919. a9ded1e5ce5d75814730bb4caaf49419 -
+
JCSE01.21 - Different Responses to Osimertinib in Primary and Acquired EGFR T790M-Mutant NSCLC Patients (ID 14711)
11:15 - 11:15 | Author(s): Baohui Han
- Abstract
Background
Primary EGFR T790M could be occasionally identified by routine molecular testing in tyrosine kinase inhibitor TKI-naive non-small cell lung cancer (NSCLC) patients. This study was aimed to compare clinical characteristics of primary and acquired T790M mutations and their responses to Osimertinib in NSCLC patients.
We collected clinical characteristics of patients diagnosed with epidermal growth factor receptor (EGFR) mutation from 2012 to 2017 in Shanghai Chest Hospital. For patients with primary and acquired T790M mutations, the responses to Osimertinib were analyzed.Primary T790M was identified in 1.03% (61/5900) of TKI-naive patients. Acquired T790M was detected in 45.50% (96/211) of TKI-treated patients. T790M always coexisted with sensitizing EGFR mutations. Primary T790M was always coexisted with 21L858R (45/61) whereas acquired T790M was coexisted with 19del (61/96). Among them, 18 patients with primary T790M mutation acquired Osimertinib and 75 patients with acquired T790M mutation received Osimertinib. The median progression-free survival (mPFS) of Osimertinib in primary T790M group was greatly longer than that in acquired T790M group (18.0 months:95% CI:15.0-21.0 VS 10.0months:95% CI:8.3-11.7, P=0.016). The DCR of both groups were 89.3% and 100%. In primary T790M group, the mPFS of concomitant occurrence of 20 T790M and 21 L858R or 19del were 15.7m and 24.0 m, respectively. In acquired T790M group, the mPFS of concomitant occurrence of 20 T790M and 21 L858R or 19del were 11.0m and 10.0m, respectively.Primary and acquired T790M-mutation patients showed different molecular characteristics. Both of them may respond to Osimertinib. However, primary T790M patients showed greater survival benefits from Osimertinib than acquired T790M patients.
a9ded1e5ce5d75814730bb4caaf49419
-
+
MA01 - Early Stage Lung Cancer: Questions and Controversies (ID 894)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Treatment of Early Stage/Localized Disease
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 10:30 - 12:00, Room 202 BD
-
+
MA01.01 - Proposal on Incorporating Lymphovascular Invasion as a T-Descriptor for Stage I Non-Small Cell Lung Cancer (ID 12754)
10:30 - 10:35 | Author(s): Baohui Han
- Abstract
- Presentation
Background
Lymphovascular invasion (LVI) and Visceral Pleural Invasion(VPI) have been reported to be risk factors for stage I Non-Small Cell Lung Cancer (NSCLC). However, only VPI was incorporated into the current 8th Tumor–Node–Metastasis(TNM) classification. This study aimed at exploring the prognostic impact of LVI on TNM staging in Pathological Stage I NSCLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively reviewed 2600 consecutive p-stage I NSCLC patients in the Shanghai Chest Hospital (2008-2012). By using the Kaplan–Meier method and Cox proportional hazard regression model, we identified the correlations between LVI, VPI and clinical outcomes in p-stage I NSCLC.
4c3880bb027f159e801041b1021e88e8 Result
Of all p-stage I NSCLC 2600 patients, 221 were pathologically diagnosed with LVI and 815 pathologically with VPI, respectively. It was observed that patients with LVI had an unfavorable lung cancer specific survival (LCSS) (hazard ratio [HR]: 1.883; 95% confidence interval [CI]: 1.351-2.625; P < 0.001) and recurrence-free survival (RFS) (HR: 2.025; 95% CI: 1.560-2.630; P < 0.001). The 5-year RFS rates of patients with LVI was significantly worse than those without LVI (61.2% VS 82.7%, P< 0.001). Patients with LVI exhibit similar prognosis (HR: 2.538; 95% CI: 1.570-4.098; P < 0.001) compared with that of VPI in pN0 non-small-cell lung cancer and a tumor diameter of 3cm or smaller. When tumor size was between 3-4cm, patients with LVI and VPI were associated with inferior prognosis than those with only LVI or VPI (P < 0.001).
8eea62084ca7e541d918e823422bd82e Conclusion
The presence of LVI independently and significantly affects LCSS and RFS in patients with stage I NSCLC. Our results suggest that stage T1a-1c(IA) patients with LVI should be upstaged to T2a(IB), meanwhile, stage T2a(IB) patients coexist with LVI and VPI should be upstaged again in the TNM classification.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
MA15 - Colliding Approaches - EGFR and Immunotherapy (ID 916)
- Event: WCLC 2018
- Type: Mini Oral Abstract Session
- Track: Targeted Therapy
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 13:30 - 15:00, Room 107
-
+
MA15.07 - Different Responses to Osimertinib in Primary and Acquired EGFR T790M-Mutant NSCLC Patients (ID 12766)
14:10 - 14:15 | Author(s): Baohui Han
- Abstract
- Presentation
Background
Primary EGFR T790M could be occasionally identified by routine molecular testing in tyrosine kinase inhibitor TKI-naive non-small cell lung cancer (NSCLC) patients. This study was aimed to compare clinical characteristics of primary and acquired T790M mutations and their responses to Osimertinib in NSCLC patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
We collected clinical characteristics of patients diagnosed with epidermal growth factor receptor (EGFR) mutation from 2012 to 2017 in Shanghai Chest Hospital. For patients with primary and acquired T790M mutations, the responses to Osimertinib were analyzed.
4c3880bb027f159e801041b1021e88e8 Result
Primary T790M was identified in 1.03% (61/5900) of TKI-naive patients. Acquired T790M was detected in 45.50% (96/211) of TKI-treated patients. T790M always coexisted with sensitizing EGFR mutations. Primary T790M was always coexisted with 21L858R (45/61) whereas acquired T790M was coexisted with 19del (61/96). Among them, 18 patients with primary T790M mutation acquired Osimertinib and 75 patients with acquired T790M mutation received Osimertinib. The median progression-free survival (mPFS) of Osimertinib in primary T790M group was greatly longer than that in acquired T790M group (18.0 months:95% CI:15.0-21.0 VS 10.0months:95% CI:8.3-11.7, P=0.016). The DCR of both groups were 89.3% and 100%. In primary T790M group, the mPFS of concomitant occurrence of 20 T790M and 21 L858R or 19del were 15.7m and 24.0 m, respectively. In acquired T790M group, the mPFS of concomitant occurrence of 20 T790M and 21 L858R or 19del were 11.0m and 10.0m, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
Primary and acquired T790M-mutation patients showed different molecular characteristics. Both of them may respond to Osimertinib. However, primary T790M patients showed greater survival benefits from Osimertinib than acquired T790M patients.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA07 - Oligometastasis: What Should Be the State-Of-The-Art? (ID 905)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Oligometastatic NSCLC
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 15:15 - 16:45, Room 107
-
+
OA07.06 - Efficacy of Local Consolidative Therapy for Oligometastatic Lung Adenocarcinoma Patients Harboring EGFR Mutations. (ID 12523)
16:10 - 16:20 | Author(s): Baohui Han
- Abstract
- Presentation
Background
For oligometastatic lung adenocarcinoma patients with sensitive EGFR mutations, the role of local consolidative therapy (LCT) remains debatable. The purpose of this study was to investigate the efficacy of LCT in these patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
Advanced stage patients with oligometastatic lung adenocarcinoma who harboring EGFR mutation were identified at the Shanghai Chest Hospital from 2010 to 2016.
4c3880bb027f159e801041b1021e88e8 Result
A total of 253 patients (149 patients who received LCT plus EGFR-TKIs [combination group] and 104 patients who received EGFR-TKIs [TKI monotherapy group] were included. The median PFS time in the combination group was 14 months versus 9 months in the TKI monotherapy group (HR=0.57, 95% [CI] 0.44, 0.79, p<0.01, Figure 1 A). The median OS time in the combination group was 33 months versus 20 months in the TKI monotherapy group (HR=0.56, 95% [CI] 0.41, 0.75, p<0.01, Figure 1D). Survival benefit was independent of EGFR mutation type (PFS: 19del, p=0.02, Figure 1B; 21L858R, p<0.01, Figure 1C; OS: 19del, p=0.0189, Figure 1E; 21L858R, p<0.01, Figure 1F) and metastatic sites .

8eea62084ca7e541d918e823422bd82e Conclusion
LCT combined with TKI therapy was feasible and significantly improved PFS and OS among oligometastatic lung adenocarcinoma patients with sensitive EGFR mutations, and thus, should be considered as an important medical treatment during clinical management.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
OA13 - Therapeutics and Radiation for Small Cell Lung Cancer (ID 927)
- Event: WCLC 2018
- Type: Oral Abstract Session
- Track: Small Cell Lung Cancer/NET
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 10:30 - 12:00, Room 203 BD
-
+
OA13.03 - Anlotinib as Third-Line or Further-Line Treatment in Relapsed SCLC: A Multicentre, Randomized, Double-Blind Phase 2 Trial (ID 12102)
10:50 - 11:00 | Author(s): Baohui Han
- Abstract
- Presentation
Background
Treatment for patients with relapsed small cell lung cancer (SCLC) who failed ≥ 2 lines of chemotherapy have high unmet needs. Anlotinib is a novel TKI with highly selective inhibition effects on multi-targets, especially on VEGFR, c-Kit, PDGFR, FGFR. Here we report results of a phase 2 study of anlotinib for the third-line and further-line treatment of SCLC. (ALTER1202, NCT03059797).
a9ded1e5ce5d75814730bb4caaf49419 Method
Eligible either limited- or extensive-stage SCLC patients with disease progression after ≥ 2 lines of chemotherapy were randomized 2:1 to anlotinib or placebo (12 mg PO QD from day 1 to 14, every 3 weeks). The primary endpoint was PFS and secondary endpoints was OS, ORR, DCR, quality of life and safety.
4c3880bb027f159e801041b1021e88e8 Result
Between March 2017 and May 2018, 120 patients from 11 centers were randomized to either anlotinib arm (n=82) or placebo arm (n=38). Until the data cutoff date (30 Jun 2018), median PFS was 4.1 months (95%CI, 2.8 to 4.2 months) in anlotinib arm and 0.7 months (95% CI, 0.7 to 0.8 months) in placebo arm (HR, 0.19; 95% CI, 0.12 to 0.32, p<0.0001). OS data were not sufficiently mature for analysis. Although ORR was similar, considerable improvement in DCR was observed in anlotinib arm (71.6% vs 13.2%, p<0.0001). Treatment-related adverse events (TRAEs) occurred more frequently in anlotinib arm than that in placebo (87.7% and 74.4%). The most common TRAEs were hypertension, anorexia, fatigue, and hand-foot syndrome. Grade ≥3 TRAEs occurred in 29 (35.8%) of patients in anlotinib arm and 6 (15.4%) in placebo arm, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
ALTER 1202 study demonstrates anlotinib should be considered a treatment option for patients with relapsed SCLC who have experienced treatment failure with two lines of chemotherapy. The safety profile was consistent with the previous report and no newly adverse events were identified.
6f8b794f3246b0c1e1780bb4d4d5dc53Only Members that have purchased this event or have registered via an access code will be able to view this content. To view this presentation, please login, select "Add to Cart" and proceed to checkout. If you would like to become a member of IASLC, please click here.
-
+
P1.01 - Advanced NSCLC (Not CME Accredited Session) (ID 933)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 4
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.01-107 - The Impact of Anlotinib on Quality of Life in Patients with Advance NSCLC: Post-Hoc Analysis of a Phase III Randomized Control Trial (ALTER0303) (ID 12249)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
Anlotinib is a novel multi-target tyrosine Kinase inhibitor that inhibits VEGFR2/3, FGFR1-4, PDGFD α/β, c-Kit and Ret. In the phase Ⅲ ALTER-0303 trial (Clinical Trial Registry ID: NCT 02388919), anlotinib significantly improved overall survival versus placebo in advanced non-small-cell lung cancer (NSCLC) patients who had received at least two previous chemotherapy and epidermal growth factor receptor /anaplastic lymphoma kinase targeted therapy regimens. This study assessed quality of life (QoL) in these patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients were randomized (2:1) to anlotinib or placebo up to progression or intolerable toxicity. The QoL were assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) and the associated EORTC Quality of Life Lung Cancer Specific Module (QLQ-LC13) at baseline, end of cycle 1, end of every two cycles, and at the final visit. The analyses were conducted in the first 6 cycles. Differences in scores of 10 points or more between two arms or from baseline were considered clinically meaningful.
4c3880bb027f159e801041b1021e88e8 Result
A total of 437 patients were assigned to anlotinib (n=294) and placebo (n=143). The completion rates of the QoL questionnaires were from 69.9 % to 97.0%. Mean scores of QLQ-C30 and QLQ-LC13 subscales were similar in the anlotinib and placebo arms at baseline. Compared to placebo, anlotinib improved role functioning (at cycle 2), social functioning (at cycle 4), dyspnea (at cycle 2, 4), insomnia (at cycle 6), constipation (at cycle 2) and financial problems (at cycle 2). Only sore mouth or tongue symptom was worse in the anlotinib arm (at cycle 2, 4, 6) than in the placebo arm.
8eea62084ca7e541d918e823422bd82e Conclusion
Anlotinib improved quality of life versus placebo in advanced NSCLC patients who had received at least two previous chemotherapies. The QoL analyses provided evidence that anlotinib should be a choice for the third-line treatment or beyond in advanced NSCLC.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-108 - Management of Anlotinib-Related Adverse Events: Data From ALTER 0303 (ID 12054)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
Anlotinib is an oral tyrosine kinase inhibitor targeting VEGFR, FGFR, PDGFR and c-kit. In the phase Ⅲ ALTER-0303 trial (Clinical Trial Registry ID: NCT 02388919), anlotinib significantly improved overall survival versus placebo in advanced non-small-cell lung cancer (NSCLC) patients who had received at least two previous chemotherapy and epidermal growth factor receptor /anaplastic lymphoma kinase targeted therapy regimens. This study summarized adverse event management in this trial.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients were randomized (2:1) to anlotinib or placebo up to progression or intolerable toxicity. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 4.0) and managed by investigators. Adverse events and key strategies for preventing and managing the most common adverse events were described. Proportions were compared using the χ2 test or Fisher’s exact test, as appropriate. Two-sided values of P <0.05 were considered statistically significant. Analyses were calculated by SAS 9.4.
4c3880bb027f159e801041b1021e88e8 Result
Between February 2015, and August 2016, a total of 437 patients were randomized to anlotinib group (n=294) and placebo group (n=143). The most common anlotinb related adverse events were hypertension (64.6%), fatigue (46.3%), TSH elevation (44.6%), hand-foot syndrome (HFS) (43.2%), hypertriglyceridemia (38.8%), anorexia (38.4%). The most common anlotinib related grade ≥3 adverse events were hypertension (13.3%), HFS (3.7%), and hypertriglyceridemia (2.4%). The median onset time of hypertension, HFS, and hypertriglyceridemia were 6 days, 30 days, and 22 days respectively.
To monitor blood pressure, every patient had an electronic manometer. One hundred and eight (36.7%) patients received dihydropyridine calcium channel blockers, 79 (26.9%) patients received converting enzyme inhibitors of angiotensin /angiotensin receptor blockers, 57 (19.4%) patients received diuretics, 35 (11.9%) patients received beta-blockers. Only 3 (1.0%) patients need dose modification due to hypertension.
Prophylactic measures of HFS were recommended. Frequent emollients should be used on hands and feet to maintain skin hydration, manicure or pedicure to control calluses, protect pressure points and tender areas of feet with insole cushions, shock-absorbing soles, comfortable shoes. Seven (2.3%) patients required dose reduction due to hand-foot skin syndrome. Eleven (3.7%) patients received cortisone cream for topical therapy.
Twenty-four patients received fibrates to reduce plasma triglyceride level. Two (0.7%) patients required dose reduction due to hypertriglyceridemia.
8eea62084ca7e541d918e823422bd82e Conclusion
Anlotinb-related adverse events could be controlled by prophylactic measures, and early intervention.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-29 - Crizotinib in Advanced Lung Adenocarcinoma Patients with ALK or ROS-1 Rearrangement: Is it the Same? (ID 12929)
16:45 - 18:00 | Presenting Author(s): Baohui Han
- Abstract
Background
Crizotinib is an orally taken tyrosine kinase inhibitor (TKI) targeting both ALK and ROS1 rearrangement, which have defined two different molecular subgroup patients. The aim of the study was to compare the therapeutic efficacy of crizotinib in advanced lung adenocarcinoma patients diagnosed with ALK or ROS1 mutation.
a9ded1e5ce5d75814730bb4caaf49419 Method
Patients diagnosed with ALK (Group A) or ROS1 (Group B) mutation were identified from our standardized registration system. The effectiveness of crizotinib in eligible patients was retrospectively analyzed.
4c3880bb027f159e801041b1021e88e8 Result
A total of 5348 and 4695 patients were screened and 393 (7.3%, 95% CI, 6.6%-8.0%) and 105 (2.2%, 95% CI, 1.8%-2.7%) positive patients were identified among the two groups, respectively. There were 141 and 32 eligible patients were included for survival analysis. The ORR is 53.0% (95% CI, 43.0%-63.0%) in group A, without statistical significance compared with group B (71%, 95% CI, 51.2%-90.4%, P=0.11). Similar result was also observed in terms of DCR (86%, [95% CI, 79.1%-92.9%] vs. 92.0%, [95%, CI, 80.0%-100.0%], P=0.74). The median PFS in group A was 12.4 months (95% CI, 10.2 -14.7 months), which was statistically worse than patients in group B (18.2 months, 95% CI, 6.3 -29.0 months, P=0.02). The OS was too immature to analyze.
8eea62084ca7e541d918e823422bd82e Conclusion
Patients harboring ROS1 rearrangement derived better prognosis compared with these who had ALK rearrangement when treated with crizotinib.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.01-30 - Crizotinib in Advanced Non-Adenocarcinoma, NSCLC (NA-NSCLC) Patients with ALK Rearrangement: A Retrospective Study and Literature Review (ID 12942)
16:45 - 18:00 | Presenting Author(s): Baohui Han
- Abstract
Background
The aim of the study was to investigate the prevalence of anaplastic lymphoma kinase (ALK) rearrangement in non-adenocarcinoma, non-small cell lung cancer (NA-NSCLC) patients and therapeutic efficacy of crizotinib in these patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
From January 2013 to January 2017, NA-NSCLC patients who were diagnosed with ALK rearrangement were screened. The effectiveness of crizotinib in positive patients was retrospectively analyzed. A literature review was performed and eligible cases were analyzed combined with our data.
4c3880bb027f159e801041b1021e88e8 Result
A total of 1212 NA-NSCLC patients were screened during the period with 25 positive patients identified (2.1%, 95% CI, 1.3%-2.9%). A statistically higher percentage of female patients (40.0% vs. 10.4%, P< 0.01), non-smoker (72.0% vs.43.2%, P< 0.01), containing adenocarcinoma component (36.0% vs. 7.1%, P< 0.01) and advanced stage (68.0% vs. 45.6%, P=0.03) were observed in ALK positive group. The median PFS of the 9 eligible patients in our institution was 7.0 months (95% CI, 0-15.6 months). We combined our data with the sporadic cases from 10 previous case reports (total n=19) and found that the median PFS was 7.0 months (95% CI, 5.6-8.4 months).
8eea62084ca7e541d918e823422bd82e Conclusion
Our study suggested the opportunity to test ALK rearrangement in NA-NSCLC patients, especially in female, non-smoker and patients containing adenocarcinoma component. Crizotinib provides an option for the treatment of NA-NSCLC patients who diagnosed with ALK rearrangement.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.03 - Biology (Not CME Accredited Session) (ID 935)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 4
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.03-05 - Transcriptome Landscape of Lung Adenocarcinoma Patients Revealed Distinct Trajectory Patterns (ID 12025)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
The hallmarks of cancer was proposed to elucidate the common trajectory of tumors of different tissues of origin and genetic makeups, which were expected to attributed to disruption of regulatory pathways conferring survival and growth advantages to tumor tissues. However, the specific biological processes involved in each milestones in the trajectory of development of lung adenocarcinoma, particularly regarding tumor stages, remain elusive. The datasets of The Cancer Genome Atlas (TCGA) project lend potential for discovering the distinctive expressional patterns of differentiated subpopulations, and exploring the dynamic evolution of biological activities within tumor cells.
a9ded1e5ce5d75814730bb4caaf49419 Method
RNA sequencing level 3 data of 56 pairs of tissue and adjacent normal samples were obtained from TCGA. Differential expression analysis was conducted using ‘edgeR’ package across the each tumor stage. Differentially expressed genes were derived with fold change>=4 and FDR <= 0.01, followed by KEGG pathway analysis. Odd ratios (ORs) were extracted to indicate the degrees of dysregulation of each pathway.
4c3880bb027f159e801041b1021e88e8 Result
After removing non-cancer associated pathways from the 77 pathways identified as dysregulated in those samples, we arrived at 35 pathways. In “Cell cycle” and “Pathways in cancer”, ORs display positive correlation with tumor stages, and the Stage IV showed appreciably higher OR than other stages. Noteworthily, Stage IV has very high Ors in “PPAR signaling pathway”, “Renin-angiotensin system” and “p53 signaling pathway”, which represents canonical pathways implicated in cancer pathologies.
8eea62084ca7e541d918e823422bd82e Conclusion
We identified pathways that display correlation with tumor stages, although some deviations are expected to stem from differences in treatment, complicated disease, and health conditions, etc. These results display considerable linear correlation between the degree of dysregulation of cancer pathways, which promise applying RNA sequencing in characterizing the bona fide cell fate of tumor tissues.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.03-16 - Anlotinib Inhibits Angiogenesis of Refractory Advanced Non-Small Cell Lung Cancer via Blocking CCL2 Expression (ID 12406)
16:45 - 18:00 | Presenting Author(s): Baohui Han
- Abstract
Background
Anlotinib has been demonstrated to be effective in prolonging progression free survival (PFS; Anlotinib: 5.37 months vs Placebo: 1.40 months) and overall survival (OS; Anlotinib: 9.63 months vs Placebo: 6.30 months) of refractory advanced Non-Small Cell Lung Cancer (NSCLC) patients in clinical trials. Clinical evidences suggested that Anlotinib-induced anti-tumor efficacy could be attributed to anti-angiogenesis. However, the underlying molecular mechanisms and predictive biomarker of Anlotinib are still unclear.
a9ded1e5ce5d75814730bb4caaf49419 Method
437 patients with advanced NSCLC enrolled in clinical study, and 294 patients received Anlotinib therapy. Retrospectively analysis of the Anlotinib-administrated 294 NSCLC patients was performed to screen out underlying biomarker for Anlotinib-responsive patients. Transcriptome and functional assays were performed to understand the anti-tumor molecular mechanism of Anlotinib in vitro. CCL2 levels and their roles in angiogenesis were evaluated by ELISA detection, RT-qPCR quantification, and immunofluorescence assay, in vivo. Changes in serum CCL2 levels were analyzed to reveal the correlation of Anlotinib response between responders and non-responders.
4c3880bb027f159e801041b1021e88e8 Result
Anlotinib therapy is more beneficial to prolong OS for NSCLC patients harboring positive driver gene mutations, especially for patients harboring EGFRT790M mutation. Moreover, our data indicated that Anlotinib-induced cell viability downregulation, cell apoptosis induction, cell invasion inhibition, cell cycle arrest, and cell migration inhibition are associated with CCL2 levels in vitro. We demonstrated that Anlotinib inhibits angiogenesis of NCI-H1975 derived xenografts model via inhibiting CCL2 in vivo. Lastly, we found that Anlotinib-induced serum CCL2 level decreases are associated with the benefits of PFS and OS, in refractory advanced NSCLC patients (n = 28).
8eea62084ca7e541d918e823422bd82e Conclusion
Our study reports a novel anti-angiogenesis mechanism of Anlotinib via inhibiting CCL2 in NCI-H1975 derived xenografts model, and suggests the changes in serum CCL2 levels may be used to monitor and predict clinical outcome in Anlotinib-administered refractory advanced NSCLC patients. The biomarker of serum CCL2 alteration may guide precision therapy of Anlotinib for NSCLC patients at third-line or over third-line.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.03-22 - MiR-125b Plays a Tumor Suppressor Role in Inflammation-Related Non-Small Cell Lung Cancer via Repressing IGF-1 Signal Pathway (ID 11752)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
Epidemiologic data have indicated that chronic inflammation was highly associated with the pathogenesis of lung cancer. However, the molecular relations between inflammation and lung cancer have not been well understood. MicroRNAs could connect the inflammatory response with tumorigenesis through regulating their cancer-related targets. The aim of the present study was to identify the core miRNA in inflammation-related lung cancer and its potential mechanisms.
a9ded1e5ce5d75814730bb4caaf49419 Method
RT-PCR was used to detect the expression of miRNAs and mRNAs. CKK8 and flow cytometry assays was performed for the function experiments. Microarray analysis and IPA analysis were used to predict the potential signal pathway.
4c3880bb027f159e801041b1021e88e8 Result
Mir-125b was the most dramatically up-regulated miRNAs after treated with IFN-r, whereas after stimulated with IL-10, mir-125b was the most strikingly down-regulated ones. Restoration of mir-125b expression could completely overrode the impact of IL-10 on both cell proliferation and apoptosis in NSCLC cell lines(Figure1). And the level of mir-125b was significantly lower in 30 NSCLC tumor tissues compared with normal controls (P<0.0001). Microarray analysis found 69 up-regulated genes and 105 down-regulated genes after down-regulate mir-125b. And IPA analysis indicated that IGF-1 signaling pathway was dramatically activated. The results were validated by RT-PCR.
8eea62084ca7e541d918e823422bd82e Conclusion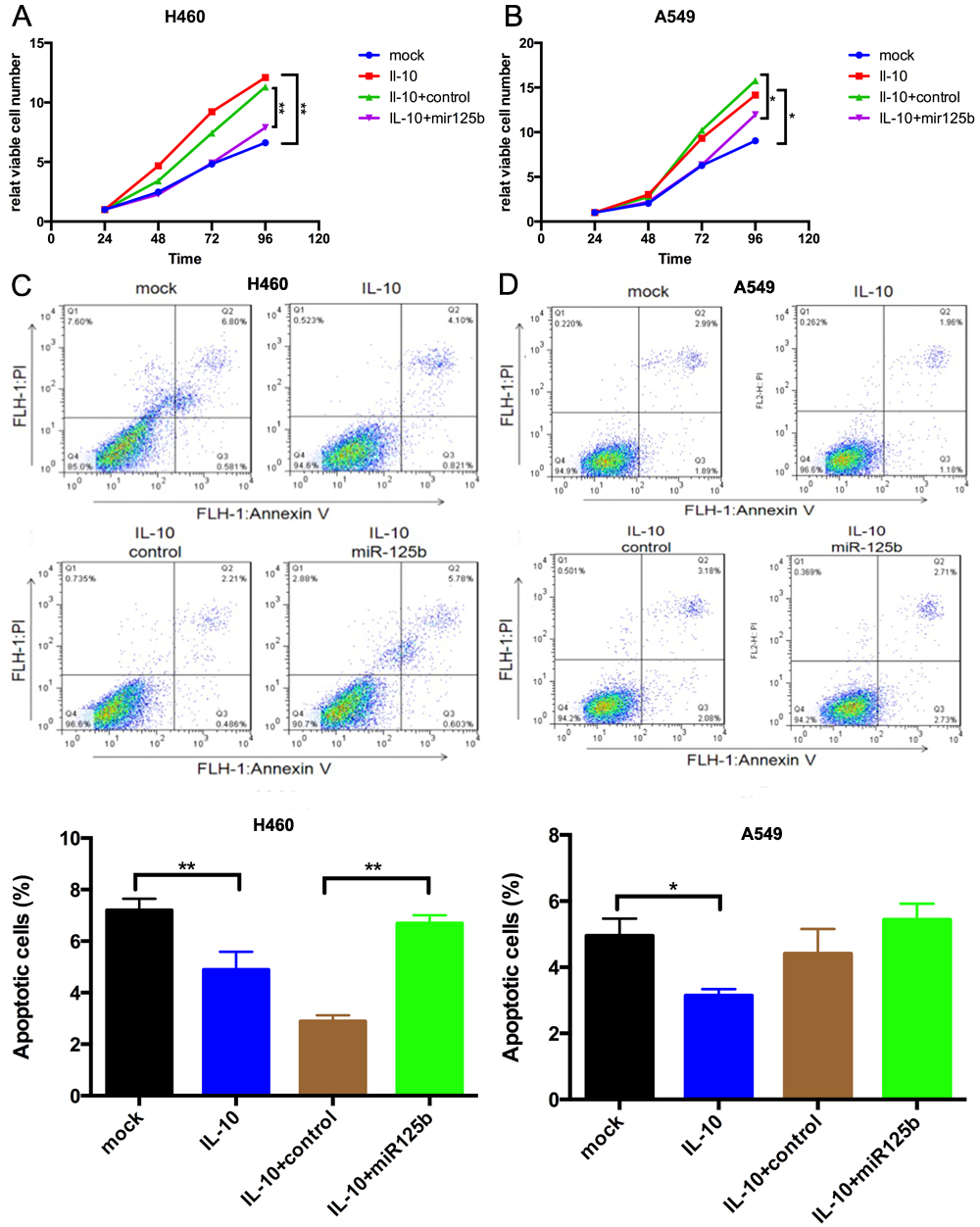
MiR-125b might play a tumor suppressor role via inhibiting IGF-1 signaling in inflammation-related lung cancer.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P1.03-30 - The Number of Mutated Repair Genes as Predictor for Tumor Mutation Burden of Lung Adenocarcinoma (ID 12027)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
Disruption of repair gene products will result in higher risk of mutation events and genetic instability. Despite some repair genes such as BRCA1 and FANCA being intensively reported in breast cancer, ovarian cancer, and the predisposition to cancers, the effects of protein dysfunction of repair genes on mutation events have not been quantified. The established repair pathways are responsible for different mutation events and may account for respective mutation patterns. Therefore, we conducted an in-depth investigation of effect of individual repair pathways or individual repair genes on tumor mutation burden (TMB).
a9ded1e5ce5d75814730bb4caaf49419 Method
We obtained level 4 variant datasets from The Cancer Genome Atlas (TCGA) which comprises of 568 samples. The TMB of each individual was calculated and the population was divided into subgroups as per the status of harboring mutations in repair genes as well as the specific repair pathways.
4c3880bb027f159e801041b1021e88e8 Result
In the 568 lung adenocarcinoma patients, 434 patients have somatic mutations in any of the 112 DNA repair genes. The individuals harboring mutations in repair genes have significantly higher TMB (Mean=3.019, S.E.=0.206) than those do not (Mean=11.085, S.E.=0.493), and we derived a 3.81-fold increase in TMB for mutations occuring in an additional repair gene. Those that harbor mutations in TP53 account for 63% of the population, and ATM and PRKDC account for 11% and 10, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
We identified most highly mutated repair genes and quantified the increase in risk for each additional mutated repair gene. Although the TMB of individuals with mutations in specific repair gene or pathway show no significant difference, a larger dataset that comprises adequate number of samples within each explanatory variables such as incidence of cell division, tumor stages to be taken into model, can be expected to derive a more robust predictor.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P1.11 - Screening and Early Detection (Not CME Accredited Session) (ID 943)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/24/2018, 16:45 - 18:00, Exhibit Hall
-
+
P1.11-19 - Expression of TNFRII in Serum is Correlated with the Significant Risk of Subcentimeter Lung Adenocarcinoma (ID 12954)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
With the rapid advances of low-dose computed tomography (LDCT) screening for lung cancer, the opportunity to detect subcentimeter non-small cell lung cancer (NSCLC) is gradually increasing. The results of many previous studies have shown that even subcentimeter NSCLCs are not always in the early stage. Thus, it is quite important for us to judge the possibility of malignancy for these patients, even the tumor size is less than 10mm. However, subcentimeter lung cancer is hard to diagnose only via biopsy and imaging features because of its tinny size. Chronic inflammation is well established as a hallmark in lung carcinogenesis. In our previous study, B lymphocyte chemoattractant (BLC), is found to be slightly associated with the risk of subcentimeter lung adenocarcinoma. The aim of the present study is to evaluate the correlation between TNF receptor type II (TNFRII) and the risk for subcentimeter lung adenocarcinoma, and the efficacy of diagnosing subcentimeter lung cancer after combining TNFRII and BLC.
a9ded1e5ce5d75814730bb4caaf49419 Method
Inflammatory biomarkers were measured in 71 subcentimeter lung adenocarcinoma patients and 71 age-, sex- and smoking-matched healthy controls by using the Luminex bead-based assay.
4c3880bb027f159e801041b1021e88e8 Result
The mean (standard deviation or SD) age of patients was 56.01 (8.91) years, and 73.20% of them were female patients (n=52). Never smokers accounted for 85.96% of patients (n=57). The expression level of TNFRII is significantly down-regulated in subcentimeter lung adenocarcinoma patients compared with the healthy controls (P<0.001). And the results were validated by oncomine data mining analysis.
Elevated levels of TNFRII were associated with an 89% reduced risk for subcentimeter lung adenocarcinoma. (OR=0.11, 95% CI: 0.04-0.30, P=2.4*10-5). BLC was associated with a 2.70-fold (95% CI: 1.31-5.58, P=7.0*10-3) increased risk of subcentimeter lung adenocarcinoma for the comparison of patients in the higher-level group with the lower-level group. To yield more information, the BLC/TNFRII ratio was created to examine their prediction for the risk of subcentimeter lung adenocarcinoma, and as expected there was a 35- fold increased risk for patients in the higher-level group relative to patients in the lower-level group. Further ROC curve analysis revealed that TNFRII was a significant diagnostic biomarker for subcentimeter lung adenocarcinoma, with the area under the curve of 0.73 (95% CI: 0.65-0.82, P=2.0*10-6). The sensitivity, specificity and accuracy were 0.75, 0.72 and 0.73, respectively.
8eea62084ca7e541d918e823422bd82e Conclusion
Our findings demonstrated that TNFRII was associated with the significant risk of subcentimeter lung adenocarcinoma, and could be a promising biomarker for accessorily diagnosing subcentimeter lung adenocarcinoma.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.01 - Advanced NSCLC (Not CME Accredited Session) (ID 950)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.01-01 - The Impact of Anlotinib on Brain Metastases of NSCLC: Post-Hoc Analysis of a Phase III Randomized Control Trial (ALTER0303) (ID 12454)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
Few evidence has measured the intracranial impact of multitargeted VEGFR-TKIs. Anlotinib hydrochloride, which targets VEGFR, PDGFR, FGFR and c-Kit, has been shown to significantly prolong PFS and OS compared with placebo as second/third-line treatment for NSCLC in a randomized, double-blind, phase Ⅲ trial (NCT02388919). Herein we sought to analyze anlotinib’s effect in managing brain metastases (BM) and its brain-associated toxicities.
a9ded1e5ce5d75814730bb4caaf49419 Method
The PFS/OS of anlotinib versus placebo in those with and without BM records at baseline were calculated and compared respectively. In addition, time to brain progression (TTBP), a direct indicator of intracranial control, was also compared between anlotinib and placebo. All calculations were adjusted for confounding factors, including stage, histology, driver mutation type, etc.
4c3880bb027f159e801041b1021e88e8 Result
A total of 437 patients (294 receiving anlotinib; 143 receiving placebo) were included. 97 cases (22.2%) were recorded to have BM at baseline. There were more BM cases among younger patients and those with adenocarcinoma. Both of the benefit magnitude from anlotinib over placebo were similar between BM and non-BM group in terms of PFS (BM: HR 0.19, 0.11-0.34; non-BM: HR 0.29, 0.22-0.37; interaction P=0.691) and OS (BM: HR 0.71, 0.44-1.16; non-BM: HR 0.67, 0.51-0.89: interaction P=0.789). More specifically, anlotinib was associated with significantly longer TTBP (HR 0.11, 95% CI 0.03-0.41, P=0.001; Figure1) despite all confounders, indicating a 90% reduction of brain progression risk from anlotinib. Interestingly, anlotinib was also associated with more neural toxicities (18.4% vs. 8.4%, P=0.007) and psychological symptoms (49.3% vs. 35.7%, P=0.008) compared with placebo, especially headache (P=0.01), brain edema (P=0.04) but not infarction or cerebral hemorrhage. The above results were all confirmed both in intention-to-treat and per-protocol population.
8eea62084ca7e541d918e823422bd82e Conclusion
Anlotinib can benefit NSCLC patients with brain metastases and is highly potent in managing intracranial lesions. Its special effect on brain metastases and cerebral tissues merits further investigation.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.03 - Biology (Not CME Accredited Session) (ID 952)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 2
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.03-22 - OCT4&SOX2 Specific CTLs Plus PD-1 Inhibitor Had Synergistic Effect on Killing CSC And Treating Drug-Resistant Lung Cancer Mice (ID 12402)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
This study aimed to investigate the synergistic effect of OCT4&SOX2 specific cytotoxic T lymphocytes (CTLs) and PD-1 inhibitor on killing lung cancer stem-like cells (LCSCs) and their efficacy in treating drug-resistant lung cancer (DRLC) mice.
a9ded1e5ce5d75814730bb4caaf49419 Method
OCT4&SOX2 specific CTLs and PD-1 inhibitor with differed doses were applied to treat PC9 cells and PC9 LCSCs. CCK8 assay and flow cytometry (FCM) assay with CFSE staining target cells before treatment and PE staining died cells after treatment were conducted to detect the cytotoxic activity. DRLC mice were constructed by injection of PC9 LCSCs suspension and Matrigel into left lung of SD mice. DRLC mice was randomly divided into 5 group: Control group, CMV pp65 CTLs group, OCT4&SOX2 CTLs group, PD-1 inhibitor group and OCT4&SOX2 CTLs+PD-1 inhibitor group.
4c3880bb027f159e801041b1021e88e8 Result
In vitro, Both CCK8 assay and FCM assay disclosed that OCT4&SOX2 specific CTLs plus PD-1 inhibitor presented with elevated cytotoxic activity on PC9 cells and PC9 LCSCs. In vivo, tumor volume and tumor weight were decreased, while tumor necrosis and tumor apoptosis were increased in OCT4&SOX2 CTLs group than CMV pp65 CTLs group and control group, and in OCT4&SOX2 CTLs+PD-1 inhibitor group than OCT4&SOX2 CTLs group and PD-1 inhibitor group. In addition, CD8 expression was increased while OCT4 and SOX2 expressions were decreased in OCT4&SOX2 CTLs+PD-1 inhibitor group than OCT4&SOX2 CTLs group and PD-1 inhibitor group.
8eea62084ca7e541d918e823422bd82e Conclusion
In conclusion, OCT4&SOX2 specific CTLs and PD-1 inhibitor presented with synergistic effect on killing LCSCs in vitro and treating DRLC mice in vivo.
6f8b794f3246b0c1e1780bb4d4d5dc53 -
+
P2.03-37 - The Efficiency of Octamer-4 Specific Cytotoxic T Cells Induce By CD40-B Cells in Killing Lung Cancer Stem-Like Cells (ID 12399)
16:45 - 18:00 | Author(s): Baohui Han
- Abstract
Background
This study aimed to investigate the correlation of Octamer-4 (OCT4) expression with clinicopathological features and prognosis in lung adenocarcinoma patients, and to further explore the killing effect of OCT4 specific cytotoxic T cells (CTLs) on lung cancer stem cells (LCSCs).
a9ded1e5ce5d75814730bb4caaf49419 Method
257 lung adenocarcinoma patients underwent thoracic surgery were enrolled in this study and tissue samples were obtained during the operation. OCT4 expression was detected by immunofluorescence staining assay. CD154+ feeder cells were constructed to transfect CD40-B cells, and then mixed with OCT4 antigen peptides and CD8+ T lymphocytes extracted from peripheral blood of lung adenocarcinoma patients, subsequently the OCT4 specific CTLs were co-cultured with PC9 LCSCs to detect the killing efficacy. OCT4+ phenotype was illuminated to be associated with poor differentiation, worse disease free survival (DFS) and overall survival (OS). And Cox’s analysis revealed OCT4+ was an independent predictive factor for shorter DFS and OS.
4c3880bb027f159e801041b1021e88e8 Result
CD40-B-cells with antigen presenting capacity was successfully constructed indicated by elevated CD86+, human leukocyte antigens (HLA)-A+ and CD80+ cells percentage, and OCT4 specific CTLs was successfully activated suggested by increased CD3+, CD4+ and CD8+ cells percentage as well as elevated interleukin (IL-2) and interferon (IFN)-γ expressions. OCT4 specific CTLs presented an elevated cytotoxic activity on LCSCs at percentage 75.5% ± 8.2% compared with CMV pp65 CTLs (25.6% ± 5.1%) and blank control CTLs (20% ± 4.7%).
8eea62084ca7e541d918e823422bd82e Conclusion
In conclusion, OCT4 expression could be served as a convincing risk biomarker for prognosis in lung adenocarcinoma patients and potential target of CTLs as immuntherapy in killing LCSCs.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.11 - Screening and Early Detection (Not CME Accredited Session) (ID 960)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.11-01 - Blood Transcriptomics Enables Detection of Pre-Invasive & Minimally-Invasive Lung Adenocarcinoma (ID 12959)
16:45 - 18:00 | Presenting Author(s): Baohui Han
- Abstract
Background
Although the low-dose computed tomography scan has been proved a useful tool for lung cancer screening, its highly false positive rate that usually over 90% limits its effectiveness for early detection of lung cancer. There is an urgent need to develop a non-invasive and cost-effective method to detect lung cancer at early stage.
a9ded1e5ce5d75814730bb4caaf49419 Method
Peripheral blood samples were collected from patients pathologically diagnosed pre-invasive or minimally-invasive lung adenocarcinomas and healthy volunteers who reported with no any pulmonary disorders. Total mRNA from peripheral whole blood was processed according to PAXgene Blood RNA Kit protocol. In this study, we compared blood gene expression data from 95 samples using microarray analysis. Quantitative PCR was then used to validate biomarker candidates identified by differential expression analysis in microarray hybridization (N = 251). The gene panel finally selected was validated in an independent population (N = 54) using quantitative PCR. Logistic regression was performed on multiple combinations of common probe sets, and data were evaluated in terms of discrimination by computing the area under the receiving operator characteristic curve.
4c3880bb027f159e801041b1021e88e8 Result
The lung cancer-specific gene signatures were identified to construct predictive model based on 6-gene panel such as HSP90AA1, UQCRQ, NDUFB2, RPL24, CKLF and GLRX, which correctly classified 29 of 39 pre-invasive or minimally-invasive lung adenocarcinomas, 30 of 38 health controls with 76.6% accuracy in training set, and 7 of 8 lung cancer, 8 of 10 health controls with 83.3% accuracy in test set. Validation by quantitative PCR confirmed the Affymetrix microarray data, with 75.7% accuracy, 75.0% sensitivity, 76..6% specificity, 0.83 of area under curve (AUC) in training set, and 91.9% accuracy, 91.7% sensitivity, 92.3% specificity, 0.94 of AUC in test set. Independent validation testing confirmed these specific gene signatures did not derived from the result of random chance with 83.3% accuracy, 84.6% sensitivity and 79.3% specificity.
8eea62084ca7e541d918e823422bd82e Conclusion
Our results indicated the feasibility of blood-based genetic signatures to identify pre-invasive and minimally-invasive lung adenocarcinoma as screening for lung cancer at very early stage.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.12 - Small Cell Lung Cancer/NET (Not CME Accredited Session) (ID 961)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.12-17 - Prophylactic Cranial Irradiation Can Not Provide Survival Benefits for Resected Small Cell Lung Cancer Without Lymph Node Involvement (ID 13996)
16:45 - 18:00 | Presenting Author(s): Baohui Han
- Abstract
Background
Currently, prophylactic cranial irradiation (PCI) was recommended for all patients after resection small-cell lung cancers, even those without lymph node metastasis. However, there is no directly evidence supporting this recommendation. The purpose of the present study is to assess the role of PCI for this subset of patients.
a9ded1e5ce5d75814730bb4caaf49419 Method
We retrospectively identified completely resected SCLC without lymph node involvement (N0M0) at the Shanghai Chest Hospital between January 2006 and May 2017.
4c3880bb027f159e801041b1021e88e8 Result
A total of 146 patients (44 patients received PCI, 102 patients did not) were identified in the study. During the observation period, 8.8 % (9/102) patients in the non-PCI-treated cohort and 11.4 % (5/44) patients in the PCI-treated cohort developed brain metastases. There was no significant difference in the risk of cerebral recurrence between the two cohorts with regard to the time to recurrence (P = 0.677). What is more, neither overall survival benefit (HR = 0.88, 95% CI: 0.47–1.65, P = 0.700) nor disease-free survival (HR = 0.95, 95% CI: 0.55–1.62, P = 0.835) was significant between the PCI-treated and non-PCI-treated cohorts.
8eea62084ca7e541d918e823422bd82e Conclusion
The present study did not support using PCI in surgically resected small cell lung cancer without lymph node involvement. A relatively lower risk of brain metastasis in this particular subset might explain the inferior efficacy of PCI.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P2.15 - Treatment in the Real World - Support, Survivorship, Systems Research (Not CME Accredited Session) (ID 964)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/25/2018, 16:45 - 18:00, Exhibit Hall
-
+
P2.15-22 - Optimal Glycemic Control Improves Prognosis for Lung Cancer Patients with Diabetes Mellitus (ID 14008)
16:45 - 18:00 | Presenting Author(s): Baohui Han
- Abstract
Background
Diabetes mellitus (DM) is a common comorbidity in patients with lung cancer (LC). This study aimed to evaluate the prognostic value of DM comorbidity for LC patients with DM and to assess whether an optimal glycemic control improves survival.
a9ded1e5ce5d75814730bb4caaf49419 Method
A total of 4390 patients diagnosed with LC between 2012 and 2013 at Shanghai Chest Hospital were retrospectively reviewed, 491 patients with DM and 3899 without DM. The relationship between hemoglobin A1c (HbA1c) level and the overal survival (OS) was plotted by a smooth curve. LC patients with DM were subdivided into the well-controlled group (HbA1c < 7%, n=438) and uncontrolled group (HbA1c ≥ 7%, n=53). OS differences among patients without DM, with well-controlled DM, and uncontrolled DM were evaluated by multivariate Cox regression analysis with adjustment for stage, sex, age, histology, smoking history and EGFR mutation status. The survival benefit of well-controlled DM was compared across subgroups.
4c3880bb027f159e801041b1021e88e8 Result
The median follow-up of the entire cohort was 35.8 months. DM patients (11.2%) had a significantly worse OS than nondiabetic patients [median (95% CI): 47.5 (39.0-56.1) vs. 73.6 (54.8-92.4) months, P<0.001]. The risk of mortality increased along with the elevation of HbA1c level. Uncontrolled DM patients tended to be male, elder, non-adenocarcinoma, with smoking history, wide-type EGFR mutations and advanced stage. Well-controlled DM patients had a worse OS [HR (95% CI): 2.3 (1.9-2.7), P<0.001] compared to nondiabetic patients without adjustment but a similar OS with adjustment for stage, sex, age, histology, smoking history and EGFR mutation status [HR (95% CI): 0.9 (0.8-1.1), P=0.185]. Benefit of well-controlled DM was more obviously seen in patients with advanced stage (III-IV) [HR (95% CI): 0.8 (0.6-1.1), P=0.130] or EGFR mutations [HR (95% CI): 1.2 (0.9-1.5), P=0.262].
8eea62084ca7e541d918e823422bd82e Conclusion
Elevated glycemic status negatively affected OS for patients with LC. LC patients with DM is recommended to have a glycemic control (HbA1c < 7%) especially for those with advanced stage and EGFR mutations.
6f8b794f3246b0c1e1780bb4d4d5dc53
-
+
P3.13 - Targeted Therapy (Not CME Accredited Session) (ID 979)
- Event: WCLC 2018
- Type: Poster Viewing in the Exhibit Hall
- Track:
- Presentations: 1
- Moderators:
- Coordinates: 9/26/2018, 12:00 - 13:30, Exhibit Hall
-
+
P3.13-09 - ALTER-0303 Study: Tumor Mutation Index (TMI) For Clinical Response to Anlotinib in Advanced NSCLC Patients at 3rd Line (ID 12395)
12:00 - 13:30 | Presenting Author(s): Baohui Han
- Abstract
Background
Anlotinib is an effective multi-targeted receptor tyrosin kinase inhibitor (TKI) for refractory advanced Non-Small Cell Lung Cancer (NSCLC) therapy at 3rd line. ALTER-0303 clinical trial has been revealed that Anlotinib significantly prolongs progression free survival (PFS; Anlotinib: 5.37 months vs Placebo: 1.40 months) and overall survival (OS; Anlotinib: 9.63 months vs Placebo: 6.30 months) with the objective response rate (ORR) of 9.18% and the disease control rate (DCR) of 80.95%. Here, we sought to understand the gene mutation determinants for clinical response to Anlotinib via next generation sequencing (NGS) upon cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA) at baseline.
a9ded1e5ce5d75814730bb4caaf49419 Method
Totally 437 advanced NSCLC patients enrolled in ALTER-0303 study, and 294 patients received Anlotinib therapy. Of the 294 patients, 80 patients were analyzed in the present study. Capture-based targeted ultradeep sequencing was performed to obtain germline and somatic mutations in cfDNA and ctDNA. Response analyses upon discovery cohort (n = 62) and validation cohort (n = 80) were performed by use of germline and somatic (G+S) mutation burden, somatic mutation burden, nonsynonymous mutation burden, and unfavorable mutation score (UMS), respectively. Based on the above independent biomarkers and their subtype factors, tumor mutation index (TMI) was developed, and then used for response analysis.
4c3880bb027f159e801041b1021e88e8 Result
Our data indicated that the patients harbouring less mutations are better response to Anlotinib therapy (G+S muatation burden, cutoff = 4000, Median PFS: 210 days vs 127 days, p = 0.0056; somatic mutation burden, cutoff = 800, Median PFS: 210 days vs 130 days; p = 0.0052; nonsynonymous mutation burden, cutoff = 50, Median PFS: 209 days vs 130 days; p = 0.0155; UMS, cutoff = 1, Median PFS: 210 days vs 131 days; p = 0.0016). TMI is an effective biomarker for Anlotinib responsive stratification (Median PFS: 210 days vs 126 days; p = 0.0008; AUC = 0.76, 95% CI: 0.62 to 0.89) upon discovery cohort and validation cohort (Median PFS: 210 days vs 127 days; p = 0.0006). Lastly, integrative analysis of TMI and IDH1 mutation suggested a more promising result for Anlotinib responsive stratification upon validation cohort (Median PFS: 244 days vs 87 days; p < 0.0001; AUC = 0.90, 95% CI: 0.82 to 0.97).
8eea62084ca7e541d918e823422bd82e Conclusion
This study provide a biomarker of TMI to stratify Anlotinib underlying responders, that may improve clinical outcome for Anlotinib therapy on refractory advanced NSCLC patients at 3rd line. Clinical trial information: NCT02388919.
6f8b794f3246b0c1e1780bb4d4d5dc53


